Population Pharmacokinetic/Pharmacodynamic Study of Linezolid in Hospital-Acquired Pneumonia Patients with Renal Insufficiency
- PMID: 39545249
- PMCID: PMC11561734
- DOI: 10.2147/DDDT.S474470
Population Pharmacokinetic/Pharmacodynamic Study of Linezolid in Hospital-Acquired Pneumonia Patients with Renal Insufficiency
Abstract
Purpose: The optimal treatment strategy in patients with hospital-acquired pneumonia (HAP) due to Gram-positive bacteria and renal insufficiency remains challenging. The objective of this study was to compare the outcomes of linezolid versus teicoplanin in HAP patients with renal insufficiency and to explore optimal dosage strategy for linezolid.
Methods: The retrospective study enrolled adult patients treated with intravenous linezolid or teicoplanin at Suzhou Municipal Hospital between July 2018 and August 2023. For the comparative pharmacodynamic study, effectiveness, safety and target attainment of trough concentration (Cmin) for teicoplanin versus linezolid treatment in HAP patients with document Gram-positive bacteria and renal insufficiency were compared. For the population pharmacokinetics (PPK) analyses, linezolid concentrations collected exclusively from HAP patients with renal insufficiency were used and the optimal dosage strategy was investigated using Monte Carlo simulations.
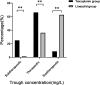
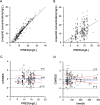
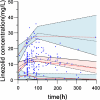
Results: Linezolid-treated patients had a higher bacterial eradication rate than teicoplanin-treated patients (88.5% vs 63.4%, P < 0.001). A higher proportion of patients in the linezolid group experienced at least one adverse reaction (42.0% vs 25.0%, P = 0.025). Significantly more supratherapeutic Cmin, less therapeutic Cmin were achieved in the linezolid group (adjusted P < 0.05). A total of 207 linezolid concentrations from 166 patients with renal insufficiency were available for the PPK analysis. Age and creatinine clearance (CrCL) were identified as significant covariates that influenced clearance. Simulations show that 300 mg q12h provide the optimal exposure in patients with a CrCL of 60 or 45 mL/min, and 200 mg q12h was recommended for patients with a CrCL of 30 or 15 mL/min.
Conclusion: Linezolid-treated patients with HAP and renal insufficiency had higher bacterial eradication rates, supratherapeutic exposure and adverse reactions than teicoplanin-treated patients. Linezolid dose reduction in patients with renal insufficiency improved the probability of achieving optimal exposure.
Keywords: hospital-acquired pneumonia; linezolid; pharmacodynamic; population pharmacokinetic; renal insufficiency.
© 2024 Xu et al.
Conflict of interest statement
We declare that there are no conflicts of interest related to the research.
Figures

Similar articles
-
Pharmacokinetics of Linezolid Dose Adjustment for Creatinine Clearance in Critically Ill Patients: A Multicenter, Prospective, Open-Label, Observational Study.Drug Des Devel Ther. 2021 May 19;15:2129-2141. doi: 10.2147/DDDT.S303497. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 34040351 Free PMC article. Clinical Trial.
-
Optimization of dosing regimens of vancomycin, teicoplanin, linezolid and daptomycin against methicillin-resistant Staphylococcus aureus in neutropenic patients with cancer by Monte Carlo simulations.J Chemother. 2021 Dec;33(8):547-553. doi: 10.1080/1120009X.2021.1931758. Epub 2021 Jun 3. J Chemother. 2021. PMID: 34080519
-
Population pharmacokinetics of teicoplanin and attainment of pharmacokinetic/pharmacodynamic targets in adult patients with haematological malignancy.Clin Microbiol Infect. 2017 Sep;23(9):674.e7-674.e13. doi: 10.1016/j.cmi.2017.02.032. Epub 2017 Mar 6. Clin Microbiol Infect. 2017. PMID: 28267636
-
A 600 mg of fixed-dose linezolid in renally impaired patients versus 15 mg/kg intermittent dose-optimized vancomycin in renally non-impaired patients: A single centre retrospective analysis for adult patients with hospital-acquired pneumonia due to methicillin-resistant Staphylococcus aureus.Trop Med Int Health. 2023 Apr;28(4):315-323. doi: 10.1111/tmi.13866. Epub 2023 Mar 23. Trop Med Int Health. 2023. PMID: 36852899 Review.
-
Therapeutic Drug Monitoring Can Improve Linezolid Dosing Regimens in Current Clinical Practice: A Review of Linezolid Pharmacokinetics and Pharmacodynamics.Ther Drug Monit. 2020 Feb;42(1):83-92. doi: 10.1097/FTD.0000000000000710. Ther Drug Monit. 2020. PMID: 31652190
References
-
- Torres A, Niederman MS, Chastre J, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociacion Latinoamericana del Torax (ALAT). Eur Respir J. 2017;50(3). doi:10.1183/13993003.00582-2017 - DOI - PubMed
-
- Antonelli A, Giani T, Coppi M, et al. Staphylococcus aureus from hospital-acquired pneumonia from an Italian nationwide survey: activity of ceftobiprole and other anti-staphylococcal agents, and molecular epidemiology of methicillin-resistant isolates. J Antimicrob Chemother. 2019;74(12):3453–3461. doi:10.1093/jac/dkz371 - DOI - PubMed
-
- Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111. doi:10.1093/cid/ciw353 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

