Parameningeal Rhabdomyosarcoma: Results of the European Pediatric Soft Tissue Sarcoma Study Group RMS 2005 Study
- PMID: 39545397
- PMCID: PMC11816552
- DOI: 10.1002/hed.27994
Parameningeal Rhabdomyosarcoma: Results of the European Pediatric Soft Tissue Sarcoma Study Group RMS 2005 Study
Abstract
Background: Parameningeal (PM) site is an unfavorable characteristic in rhabdomyosarcoma (RMS). We described the treatment and outcome for patients with PM RMS and investigated the prognostic value of risk factors. We scored PM site by originating site and by highest risk extension.
Methods: Patients with PM RMS were treated within the European pediatric Soft tissue sarcoma Study Group (EpSSG) RMS 2005 study with risk-adapted, multi-modal treatment.
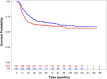
Results: Three-hundred-eighty-one patients with PM RMS were included. Radiotherapy was administered in 359 patients (77 with surgery). After a median follow-up of 75 months, 5-year event-free survival was 60% (95% confidence interval (CI) 55%-65%), 5-year overall survival was 65% (95% CI 60%-70%).
Conclusions: The outcome for patients with PM RMS has not improved in comparison to previous historical studies, despite the more rigorous application of radiotherapy (94% of patients). Signs of meningeal involvement, PM site, and age at diagnosis remained prognostic risk factors.
Trial registration: EudraCT number 2005-000217-35.
Keywords: head and neck; parameningeal; pediatric; prognostic risk factors; rhabdomyosarcoma.
© 2024 The Author(s). Head & Neck published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Bisogno G., Jenney M., Bergeron C., et al., “Addition of Dose‐Intensified Doxorubicin to Standard Chemotherapy for Rhabdomyosarcoma (EpSSG RMS 2005): A Multicentre, Open‐Label, Randomised Controlled, Phase 3 Trial,” Lancet Oncology 19 (2018): 1061–1071. - PubMed
-
- Bisogno G., De Salvo G. L., Bergeron C., et al., “Vinorelbine and Continuous Low‐Dose Cyclophosphamide as Maintenance Chemotherapy in Patients With High‐Risk Rhabdomyosarcoma (RMS 2005): A Multicentre, Open‐Label, Randomised, Phase 3 Trial,” Lancet Oncology 20 (2019): 1566–1575. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources