Insulin-like growth factor 2 drives fibroblast-mediated tumor immunoevasion and confers resistance to immunotherapy
- PMID: 39545420
- PMCID: PMC11563680
- DOI: 10.1172/JCI183366
Insulin-like growth factor 2 drives fibroblast-mediated tumor immunoevasion and confers resistance to immunotherapy
Abstract
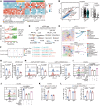
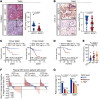
T cell exclusion is crucial in enabling tumor immune evasion and immunotherapy resistance. However, the key genes driving this process remain unclear. We uncovered a notable increase of insulin-like growth factor 2 (IGF2) in immune-excluded tumors, predominantly secreted by cancer-associated fibroblasts (CAFs). Using mice with systemic or fibroblast-specific deletion of IGF2, we demonstrated that IGF2 deficiency enhanced the infiltration and cytotoxic activity of CD8+ T cells, leading to a reduction in tumor burden. Integration of spatial and single-cell transcriptomics revealed that IGF2 promoted interaction between CAFs and T cells via CXCL12 and programmed death ligand 1 (PD-L1). Mechanistically, autocrine IGF2 activated PI3K/AKT signaling by binding to the IGF1 receptor (IGF1R) on CAFs, which was required for the immunosuppressive functions of CAFs. Furthermore, genetic ablation of IGF2 or targeted inhibition of the IGF2/IGF1R axis with the inhibitor linsitinib markedly boosted the response to immune checkpoint blockade. Clinically, elevated levels of IGF2 in tumors or plasma correlated with an adverse prognosis and reduced efficacy of anti-programmed death 1 treatment. Together, these results highlight the pivotal role of IGF2 in promoting CAF-mediated immunoevasion, indicating its potential as a biomarker and therapeutic target in immunotherapy.
Keywords: Cancer immunotherapy; Oncology.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

