Microfocused Ultrasound With Visualization Induces Remodeling of Collagen and Elastin Within the Skin
- PMID: 39545626
- PMCID: PMC11743342
- DOI: 10.1111/jocd.16638
Microfocused Ultrasound With Visualization Induces Remodeling of Collagen and Elastin Within the Skin
Abstract
Purpose: Microfocused ultrasound with real-time visualization (MFU-V) is often used for noninvasive skin lifting, by precisely targeting dermal and subcutaneous tissues to create thermal coagulation points (TCPs). These TCPs denature collagen and initiate a transient inflammatory response, ultimately attracting dermal fibroblasts and inducing efficient neocollagenesis and extracellular matrix (ECM) remodeling, yielding to MFU-V's desired skin-lifting effects. The current study investigates MFU-V's underlying mode of action based on the histological progression of TCPs in the skin, providing new insight into the technology's regenerative effect.
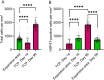
Methods: Following standard triple-depth MFU-V treatment, in vivo skin samples were assessed using histology and immunohistochemistry to evaluate TCPs, heat shock protein (HSP47), and elastin expression in fibroblasts.
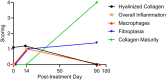
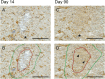
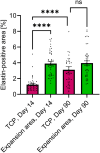
Results: MFU-V treatment induced elongated, flame-like TCPs with denatured collagen at focal depths of 1.5, 3.0, and 4.5 mm within the skin-each corresponding to its respective transducer depth. Time-dependent progression of TCPs showed significantly increased scores of fibroblasts and mature collagen along with recruitment of HSP47-positive fibroblasts to TCP areas on Day 90. Collagen formation and later maturation were visualized. Newly synthesized elastin significantly increased in the TCP area on Day 90 compared to Day 14.
Conclusion: This work provides histological evidence of stimulation and regeneration of newly synthesized elastin fibers after TCP induction. MFU-V-generated TCPs triggered the body's own healing cascade of collagen denaturation, transient inflammation, proliferation, and tissue remodeling, resulting in attraction of HSP47-positive fibroblasts to the TCP sites, and new collagen and elastin fiber regeneration by fibroblasts. Besides the well-described neocollagenesis, this study demonstrates that MFU-V treatment induces elastin neogenesis that may result not only in skin lifting but also in improved skin elasticity, providing an overall regenerative effect.
Keywords: energy‐based device (EBD); extracellular matrix (ECM); healing process; microfocused ultrasound; regeneration; thermal coagulation point (TCP).
© 2024 The Author(s). Journal of Cosmetic Dermatology published by Wiley Periodicals LLC.
Conflict of interest statement
K. Marquardt, C. Hartmann, F. Wegener, and T. Hengl were employees of Merz Aesthetics GmbH. D. Halbert and S. Hsu were employees of Merz North America, Inc. J.‐Y. Park has no financial interest to disclose in relation to the content of the article. All authors contributed to the development and review of this work, agreed with the content, and report no conflicts of interest in this work.
Figures



References
-
- Contini M., Hollander M. H. J., Vissink A., Schepers R. H., Jansma J., and Schortinghuis J., “A Systematic Review of the Efficacy of Microfocused Ultrasound for Facial Skin Tightening,” International Journal of Environmental Research and Public Health 20, no. 2 (2023): 1522, 10.3390/ijerph20021522. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

