Luminal epithelial cells integrate variable responses to aging into stereotypical changes that underlie breast cancer susceptibility
- PMID: 39545637
- PMCID: PMC11723586
- DOI: 10.7554/eLife.95720
Luminal epithelial cells integrate variable responses to aging into stereotypical changes that underlie breast cancer susceptibility
Abstract
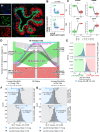
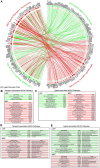
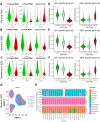
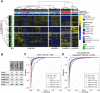
Effects from aging in single cells are heterogenous, whereas at the organ- and tissue-levels aging phenotypes tend to appear as stereotypical changes. The mammary epithelium is a bilayer of two major phenotypically and functionally distinct cell lineages: luminal epithelial and myoepithelial cells. Mammary luminal epithelia exhibit substantial stereotypical changes with age that merit attention because these cells are the putative cells-of-origin for breast cancers. We hypothesize that effects from aging that impinge upon maintenance of lineage fidelity increase susceptibility to cancer initiation. We generated and analyzed transcriptomes from primary luminal epithelial and myoepithelial cells from younger <30 (y)ears old and older >55 y women. In addition to age-dependent directional changes in gene expression, we observed increased transcriptional variance with age that contributed to genome-wide loss of lineage fidelity. Age-dependent variant responses were common to both lineages, whereas directional changes were almost exclusively detected in luminal epithelia and involved altered regulation of chromatin and genome organizers such as SATB1. Epithelial expression variance of gap junction protein GJB6 increased with age, and modulation of GJB6 expression in heterochronous co-cultures revealed that it provided a communication conduit from myoepithelial cells that drove directional change in luminal cells. Age-dependent luminal transcriptomes comprised a prominent signal that could be detected in bulk tissue during aging and transition into cancers. A machine learning classifier based on luminal-specific aging distinguished normal from cancer tissue and was highly predictive of breast cancer subtype. We speculate that luminal epithelia are the ultimate site of integration of the variant responses to aging in their surrounding tissue, and that their emergent phenotype both endows cells with the ability to become cancer-cells-of-origin and represents a biosensor that presages cancer susceptibility.
Keywords: aging; breast cancer; cancer biology; cancer susceptibility; computational biology; gene expression variance; human; lineage fidelity; lumininal epithelia; systems biology.
Plain language summary
One of the main risk factors for breast cancers is aging. But how exactly aging contributes to breast cancer is not fully understood. The mammary gland is the part of the breast that can produce milk, and is composed of two major cell types: luminal epithelial cells and myoepithelial cells. Most age-related breast cancers are thought to originate from luminal epithelial cells, but some age-related changes in myoepithelial cells may also cause cancer. These changes in breast epithelial cells vary across individuals, which may explain why some individuals are at a higher risk for breast cancer than others. Studying age-related changes in luminal and myoepithelial cells may help scientists pinpoint what causes age-related breast cancers. It may also help scientists to identify those at high risk for breast cancer, or develop alternative treatments. It could also help prevent breast cancers from occuring, getting worse or coming back. Studying age-related changes in gene expression in these two types of breast epithelial cells is the first step. Sayaman et al. showed that aging leads to widespread changes in the genes expressed by luminal and myoepithelial cells. The researchers compared luminal and myoepithelial cells from breast tissue samples taken from postmenopausal women older than 55 years and premenopausal women younger than 30 years. Their experiments and analyses revealed that age-related gene expression changes reduced the cells' ability to maintain their identity and function over time. Many of the age-related expression changes occurred in cancer-linked genes. Sayaman et al. found luminal and myoepithelial cells had increasingly varied gene expression among older women compared to younger women. Specifically, they saw changes in genes that helped these cells communicate, essentially changing the message relayed from myoepithelial cells to the neighboring luminal cells. The experiments further revealed large increases and decreases in gene expression with age in luminal cells but not in myoepithelial cells. Most of these age-related changes in luminal cells were linked to genes that play a role in cancer development. These findings suggest that age-related changes make luminal cells more prone to becoming cancerous. Sayaman et al. also developed machine learning algorithms to identify age-related gene expression patterns that may distinguish tumor cells from normal breast tissue. More research in larger populations will help confirm the results. But if these efforts are successful, they may one day help clinicians identify women at risk of age-related breast cancers, detect cancers early, or create personalized prevention or treatment approaches.
Conflict of interest statement
RS, MM, EC, PS, AZ, SS, MT, VS, SN, MS, DS, ML No competing interests declared
Figures




















Update of
- doi: 10.1101/2022.09.22.509091
Similar articles
-
Age-related gene expression in luminal epithelial cells is driven by a microenvironment made from myoepithelial cells.Aging (Albany NY). 2017 Oct 9;9(10):2026-2051. doi: 10.18632/aging.101298. Aging (Albany NY). 2017. PMID: 29016359 Free PMC article.
-
Evidence for accelerated aging in mammary epithelia of women carrying germline BRCA1 or BRCA2 mutations.Nat Aging. 2021 Sep;1(9):838-849. doi: 10.1038/s43587-021-00104-9. Epub 2021 Sep 14. Nat Aging. 2021. PMID: 35187501 Free PMC article.
-
Transcriptome analysis of mammary epithelial subpopulations identifies novel determinants of lineage commitment and cell fate.BMC Genomics. 2008 Dec 8;9:591. doi: 10.1186/1471-2164-9-591. BMC Genomics. 2008. PMID: 19063729 Free PMC article.
-
Myoepithelial cells: their origin and function in breast morphogenesis and neoplasia.J Mammary Gland Biol Neoplasia. 2005 Jul;10(3):261-72. doi: 10.1007/s10911-005-9586-4. J Mammary Gland Biol Neoplasia. 2005. PMID: 16807805 Free PMC article. Review.
-
Do myoepithelial cells hold the key for breast tumor progression?J Mammary Gland Biol Neoplasia. 2005 Jul;10(3):231-47. doi: 10.1007/s10911-005-9584-6. J Mammary Gland Biol Neoplasia. 2005. PMID: 16807803 Review.
Cited by
-
Reciprocal immune-epithelial interaction during breast cancer induction.J Immunother Cancer. 2025 Jun 19;13(6):e011453. doi: 10.1136/jitc-2024-011453. J Immunother Cancer. 2025. PMID: 40537241 Free PMC article. Review.
-
Comprehensive single-cell aging atlas of healthy mammary tissues reveals shared epigenomic and transcriptomic signatures of aging and cancer.Nat Aging. 2025 Jan;5(1):122-143. doi: 10.1038/s43587-024-00751-8. Epub 2024 Nov 25. Nat Aging. 2025. PMID: 39587369 Free PMC article.
-
DNA Methylation Concurrence, Independent of DNA Methylation Ratios, Is Associated with Chromatin Accessibility and 3D Genome Architecture.Int J Mol Sci. 2025 Jul 25;26(15):7199. doi: 10.3390/ijms26157199. Int J Mol Sci. 2025. PMID: 40806343 Free PMC article.
-
Tissue specificity of ageing in cancer risk prediction.Nat Rev Cancer. 2025 Mar 13. doi: 10.1038/s41568-025-00806-x. Online ahead of print. Nat Rev Cancer. 2025. PMID: 40082709 No abstract available.
-
Ageing, immune fitness and cancer.Nat Rev Cancer. 2025 Aug 14. doi: 10.1038/s41568-025-00858-z. Online ahead of print. Nat Rev Cancer. 2025. PMID: 40813902 Review.
References
-
- Abel GJ. Migest: methods for the indirect estimation of bilateral migration. 1.8.1R Package. 2019 https://CRAN.R-project.org/package=migest
-
- Barbie DA, Tamayo P, Boehm JS, Kim SY, Moody SE, Dunn IF, Schinzel AC, Sandy P, Meylan E, Scholl C, Fröhling S, Chan EM, Sos ML, Michel K, Mermel C, Silver SJ, Weir BA, Reiling JH, Sheng Q, Gupta PB, Wadlow RC, Le H, Hoersch S, Wittner BS, Ramaswamy S, Livingston DM, Sabatini DM, Meyerson M, Thomas RK, Lander ES, Mesirov JP, Root DE, Gilliland DG, Jacks T, Hahn WC. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;462:108–112. doi: 10.1038/nature08460. - DOI - PMC - PubMed
MeSH terms
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
- R01 CA237602/CA/NCI NIH HHS/United States
- K01 CA279498/CA/NCI NIH HHS/United States
- U01 CA244109/CA/NCI NIH HHS/United States
- T32CA221709/CA/NCI NIH HHS/United States
- DE-AC02-05CH11231/United States Department of Energy
- R01CA237602/CA/NCI NIH HHS/United States
- BC181737/Department of Defense
- R01EB024989/EB/NIBIB NIH HHS/United States
- R01 EB024989/EB/NIBIB NIH HHS/United States
- P30 CA033572/CA/NCI NIH HHS/United States
- T32 CA221709/CA/NCI NIH HHS/United States
- U01CA244109/CA/NCI NIH HHS/United States
- R33AG059206/AG/NIA NIH HHS/United States
- BC141351/Department of Defense
- K01CA279498/CA/NCI NIH HHS/United States
- R33 AG059206/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

