Polmacoxib 2mg in patients with mild to moderate idiopathic osteoarthritis of hip/knee-a randomized, double-anonymous study
- PMID: 39545640
- PMCID: PMC11721844
- DOI: 10.1080/17581869.2024.2427944
Polmacoxib 2mg in patients with mild to moderate idiopathic osteoarthritis of hip/knee-a randomized, double-anonymous study
Abstract
Aim: Polmacoxib, a new COX-2 inhibitor with carbonic anhydrase (CA) inhibitory action, is expected to help minimize the adverse effects associated with other NSAIDs, like GI (gastrointestinal) and CV (cardiovascular) system- related issues. The comparative efficacy and safety of polmacoxib 2 mg (manufactured by Hetero Labs Limited) versus celecoxib 200 mg (manufactured by Hetero Labs Limited) were assessed in this randomized, double-anonymous, clinical study in Indian adult patients diagnosed with idiopathic osteoarthritis (OA) of the.
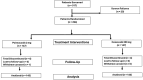
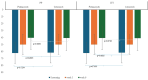
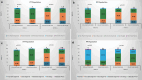
Patients & methodology: 18 years and older patients of either sex, clinically and radiographically diagnosed idiopathic knee or hip OA were randomized to receive either polmacoxib or celecoxib in a 1:1 ratio. All patients were assessed with various pain measuring scales and recorded the scores at the end of weeks 3 and 6.
Conclusion: The data for all the pain assessment scores were analyzed, and polmacoxib was found to be a non-inferior therapeutic agent compared to celecoxib in terms of safety and efficacy.(https://ctri.nic.in/CTRI/2022/05/042923).
Keywords: COX-2 inhibitor; Osteoarthritis; WOMAC pain subscale; pain management; polmacoxib.
Plain language summary
Gastric and cardiovascular system- related issues are the expected unwanted effects with the use of pain killers. Polmacoxib, a new drug for pain with dual action and lesser side effects, is expected to help in minimizing the unwanted effects like with other NSAIDs. The efficacy and safety of polmacoxib versus celecoxib were assessed in this clinical study in Indian adult patients with osteoarthritis (OA) of the hip or knee with no known reason. Adult male and female patients with knee or hip OA were taken into the study, polmacoxib or celecoxib were given to the patients in equal ratio, after obtaining a consent, which explains about the untoward effects of the treatment drugs and other study details. The severity of the pain in all the patients was estimated with standard pain measuring scales and recorded the scores at the end of weeks 3 and 6. Conclusion: The data for all the pain assessment scores were analyzed, and polmacoxib was found to be a non-inferior therapeutic agent compared to celecoxib in terms of safety and efficacy.
Conflict of interest statement
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures


References
-
- Garcia Rodriguez LA, Jick H.. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet. 1994;343(8900):769–772. doi: 10.1016/s0140-6736(94)91843-0 - DOI - PubMed
-
• Reason for the reference: Gastric bleeding due to the usage of NSAIDs and need of alternate therapy for pain management without gastrointestinal adverse effects.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
