Plant organic nitrogen nutrition: costs, benefits, and carbon use efficiency
- PMID: 39545649
- PMCID: PMC11711965
- DOI: 10.1111/nph.20285
Plant organic nitrogen nutrition: costs, benefits, and carbon use efficiency
Abstract
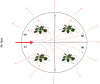
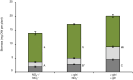
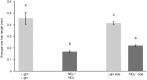
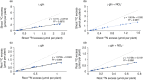
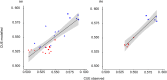
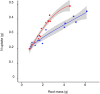
Differences in soil mobility and assimilation costs between organic and inorganic nitrogen (N) compounds would hypothetically induce plant phenotypic plasticity to optimize acquisition of, and performance on, the different N forms. Here we evaluated this hypothesis experimentally and theoretically. We grew Arabidopsis in split-root setups combined with stable isotope labelling to study uptake and distribution of carbon (C) and N from l-glutamine (l-gln) and NO3 - and assessed the effect of the N source on biomass partitioning and carbon use efficiency (CUE). Analyses of stable isotopes showed that 40-48% of C acquired from l-gln resided in plants, contributing 7-8% to total C of both shoots and roots. Plants grown on l-gln exhibited increased root mass fraction and root hair length and a significantly lower N uptake rate per unit root biomass but displayed significantly enhanced CUE. Our data suggests that organic N nutrition is linked to a particular phenotype with extensive growth of roots and root hairs that optimizes for uptake of less mobile N forms. Increased CUE and lower N uptake per unit root growth may be key facets linked to the organic N phenotype.
Keywords: Arabidopsis thaliana; amino acids; carbon use efficiency; glutamine; organic nitrogen; root hair.
© 2024 The Author(s). New Phytologist © 2024 New Phytologist Foundation.
Conflict of interest statement
TN declares a competing interest as he owns shares in, and works part time for, the company Arevo AB that develops, produces, and markets organic fertilizers. RG also declares a competing interest as she is also employed by Arevo AB. All other authors declare that the research was performed without any conflicting commercial or financial relationships and hence declare no conflict of interest.
Figures


References
-
- Ågren GI, Ingestad T. 1987. Root:shoot ratio as a balance between nitrogen productivity and photosynthesis. Plant, Cell & Environment 10: 579–586.
-
- Bates TR, Lynch JP. 2001. Root hairs confer a competitive advantage under low phosphorus availability. Plant and Soil 236: 243–250.
-
- Bhat KKS, Nye PH, Bereton AJ. 1979. The possibility of predicting solute uptake and plant growth responses from independently measured soil and plant characteristics. VI. The growth and uptake of rape in solution of constant nitrate concentration. Plant and Soil 53: 137–167.
-
- Bienert MD, Werner KM, Wimmer MA, Bienert GP. 2021. Root hairs: the villi of plants. Biochemical Society Transactions 49: 1133–1146. - PubMed
-
- Bloom AJ, Meyerhoff PA, Taylor AR, Rost TL. 2003. Root development and the absorption of ammonium and nitrate from the rhizosphere. Journal of Plant Growth Regulation 21: 416–431.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

