Protective threshold of a potent neutralizing Zika virus monoclonal antibody in rhesus macaques
- PMID: 39545728
- PMCID: PMC11650990
- DOI: 10.1128/jvi.01429-24
Protective threshold of a potent neutralizing Zika virus monoclonal antibody in rhesus macaques
Abstract
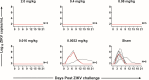
Zika virus (ZIKV) is a mosquito-borne flavivirus that caused a global pandemic in 2016-2017 with continued ongoing transmission at low levels in several countries. In the absence of an approved ZIKV vaccine, neutralizing monoclonal antibodies (mAbs) provide an option for the prevention and treatment of ZIKV infection. Previous studies identified a potent neutralizing human mAb ZIKV-117 that reduced fetal infection and death in mice following ZIKV challenge. In this study, we report exquisite potency of ZIKV-117-LALA-YTE, which has been engineered to reduce Fc receptor binding and to extend half-life, in a titration study in rhesus macaques to protect against ZIKV challenge. We show complete protection at a dose of 0.016 mg/kg ZIKV-117-LALA-YTE, which resulted in median serum concentrations of 0.13 µg/mL. The high potency of this mAb supports its potential clinical development as a novel biotherapeutic intervention for ZIKV.IMPORTANCEIn this study, we report the potency of the Zika virus (ZIKV)-specific neutralizing antibody ZIKV-117-LALA-YTE against ZIKV challenge in a titration study rhesus macaques. This high potency supports the further development of this monoclonal antibody for ZIKV.
Keywords: Zika; antibody; biotherapeutic; efficacy; non-human primate.
Conflict of interest statement
J.E.C. has served as a consultant for Luna Labs USA, Merck Sharp & Dohme Corporation, and Emergent Biosolutions; is a former member of the Scientific Advisory Boards of Gigagen (Grifols), Meissa Vaccines, and BTG International; is founder of IDBiologics; and receives royalties from UpToDate. The laboratory of J.E.C. received unrelated sponsored research agreements from AstraZeneca, Takeda Vaccines, and IDBiologics during the conduct of the study. All other authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

