Animal models for exploring Chagas disease pathogenesis and supporting drug discovery
- PMID: 39545730
- PMCID: PMC11629624
- DOI: 10.1128/cmr.00155-23
Animal models for exploring Chagas disease pathogenesis and supporting drug discovery
Abstract
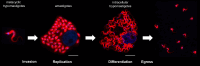
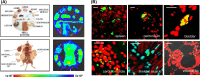
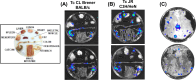
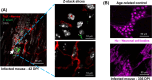
SUMMARYInfections with the parasitic protozoan Trypanosoma cruzi cause Chagas disease, which results in serious cardiac and/or digestive pathology in 30%-40% of individuals. However, symptomatic disease can take decades to become apparent, and there is a broad spectrum of possible outcomes. The complex and long-term nature of this infection places a major constraint on the scope for experimental studies in humans. Accordingly, predictive animal models have been a mainstay of Chagas disease research. The resulting data have made major contributions to our understanding of parasite biology, immune responses, and disease pathogenesis and have provided a platform that informs and facilitates the global drug discovery effort. Here, we provide an overview of available animal models and illustrate how they have had a key impact across the field.
Keywords: Chagas disease; Trypanosoma cruzi; animal models; drug development; pathogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Zingales B, Andrade SG, Briones MRS, Campbell DA, Chiari E, Fernandes O, Guhl F, Lages-Silva E, Macedo AM, Machado CR, Miles MA, Romanha AJ, Sturm NR, Tibayrenc M, Schijman AG, Second Satellite Meeting . 2009. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz 104:1051–1054. doi: 10.1590/s0074-02762009000700021 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

