Targeting resident astrocytes attenuates neuropathic pain after spinal cord injury
- PMID: 39545839
- PMCID: PMC11567666
- DOI: 10.7554/eLife.95672
Targeting resident astrocytes attenuates neuropathic pain after spinal cord injury
Abstract
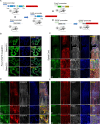
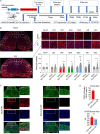
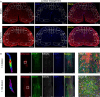
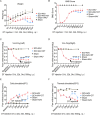
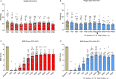
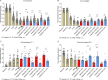
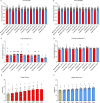
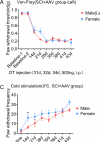
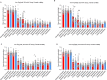
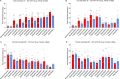
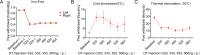
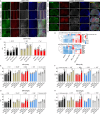
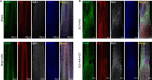
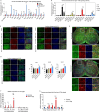
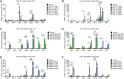
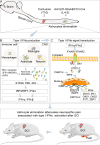
Astrocytes derive from different lineages and play a critical role in neuropathic pain after spinal cord injury (SCI). Whether selectively eliminating these main origins of astrocytes in lumbar enlargement could attenuate SCI-induced neuropathic pain remains unclear. Through transgenic mice injected with an adeno-associated virus vector and diphtheria toxin, astrocytes in lumbar enlargement were lineage traced, targeted, and selectively eliminated. Pain-related behaviors were measured with an electronic von Frey apparatus and a cold/hot plate after SCI. RNA sequencing, bioinformatics analysis, molecular experiment, and immunohistochemistry were used to explore the potential mechanisms after astrocyte elimination. Lineage tracing revealed that the resident astrocytes but not ependymal cells were the main origins of astrocytes-induced neuropathic pain. SCI-induced mice to obtain significant pain symptoms and astrocyte activation in lumbar enlargement. Selective resident astrocyte elimination in lumbar enlargement could attenuate neuropathic pain and activate microglia. Interestingly, the type I interferons (IFNs) signal was significantly activated after astrocytes elimination, and the most activated Gene Ontology terms and pathways were associated with the type I IFNs signal which was mainly activated in microglia and further verified in vitro and in vivo. Furthermore, different concentrations of interferon and Stimulator of interferon genes (STING) agonist could activate the type I IFNs signal in microglia. These results elucidate that selectively eliminating resident astrocytes attenuated neuropathic pain associated with type I IFNs signal activation in microglia. Targeting type I IFNs signals is proven to be an effective strategy for neuropathic pain treatment after SCI.
Keywords: Type I interferon; astrocyte; mouse; neuropathic pain; neuroscience; origins; spinal cord injury.
© 2024, Zhao, Zhu, Ren et al.
Conflict of interest statement
QZ, YZ, YR, LZ, JZ, SY, HN, RZ, LC, NX No competing interests declared
Figures



Update of
- doi: 10.1101/2024.01.23.576887
- doi: 10.7554/eLife.95672.1
- doi: 10.7554/eLife.95672.2
Similar articles
-
IL-6/JAK2/STAT3 axis mediates neuropathic pain by regulating astrocyte and microglia activation after spinal cord injury.Exp Neurol. 2023 Dec;370:114576. doi: 10.1016/j.expneurol.2023.114576. Epub 2023 Oct 18. Exp Neurol. 2023. PMID: 37863306
-
Truncated TrkB.T1-Mediated Astrocyte Dysfunction Contributes to Impaired Motor Function and Neuropathic Pain after Spinal Cord Injury.J Neurosci. 2017 Apr 5;37(14):3956-3971. doi: 10.1523/JNEUROSCI.3353-16.2017. Epub 2017 Mar 7. J Neurosci. 2017. PMID: 28270575 Free PMC article.
-
Mechanisms and Therapeutic Prospects of Microglia-Astrocyte Interactions in Neuropathic Pain Following Spinal Cord Injury.Mol Neurobiol. 2025 Apr;62(4):4654-4676. doi: 10.1007/s12035-024-04562-1. Epub 2024 Oct 29. Mol Neurobiol. 2025. PMID: 39470872 Review.
-
Estrogen alleviates neuropathic pain induced after spinal cord injury by inhibiting microglia and astrocyte activation.Biochim Biophys Acta Mol Basis Dis. 2018 Jul;1864(7):2472-2480. doi: 10.1016/j.bbadis.2018.04.006. Epub 2018 Apr 16. Biochim Biophys Acta Mol Basis Dis. 2018. PMID: 29653184
-
The dual role of microglia in neuropathic pain after spinal cord injury: Detrimental and protective effects.Exp Neurol. 2023 Dec;370:114570. doi: 10.1016/j.expneurol.2023.114570. Epub 2023 Oct 16. Exp Neurol. 2023. PMID: 37852469 Review.
References
MeSH terms
Substances
Associated data
Grants and funding
- 81810001048/International cooperation project of National Natural Science Foundation of China
- 81974190/Natural Science Foundation of China
- 82271419/Natural Science Foundation of China
- 82225027/Natural Science Foundation of China
- 81901902/Natural Science Foundation of China
- 81701217/Natural Science Foundation of China
- 2020YFC2008703/National Key R&D Program of China
- 22QA1408200/Shanghai Rising-Star Program
- 22120220555/Fundamental Research Funds for the Central Universities
- 22120230138/Fundamental Research Funds for the Central Universities
- KPB1702/Programs in Emergency and Critical Care Medicine, Shanghai Municipal Health Commission
- GJ2301/National Key Specialty Program, National Health Commission of China
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

