Divergent Clinical and Immunologic Outcomes Based on STK11 Co-mutation Status in Resectable KRAS-Mutant Lung Cancers Following Neoadjuvant Immune Checkpoint Blockade
- PMID: 39545922
- PMCID: PMC11739779
- DOI: 10.1158/1078-0432.CCR-24-2983
Divergent Clinical and Immunologic Outcomes Based on STK11 Co-mutation Status in Resectable KRAS-Mutant Lung Cancers Following Neoadjuvant Immune Checkpoint Blockade
Abstract
Purpose: Co-mutations of the Kirsten rat sarcoma virus (KRAS) and serine/threonine kinase 11 (STK11) genes in advanced non-small cell lung cancer (NSCLC) are associated with immune checkpoint blockade (ICB) resistance. Although neoadjuvant chemoimmunotherapy is now a standard-of-care treatment for resectable NSCLC, the clinical and immunologic impacts of KRAS and STK11 co-mutations in this setting are unknown.
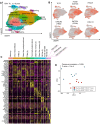
Experimental design: We evaluated and compared recurrence-free survival of resectable KRAS-mutated NSCLC tumors, with or without co-occurring STK11 mutations, treated with neoadjuvant ICB. Single-cell transcriptomics was performed on tumor-infiltrating T cells from seven KRASmut/STK11wt tumors and six KRAS and STK11 co-mutated (KRASmut/STK11mut) tumors.
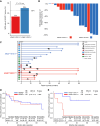
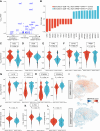
Results: Relative to KRASmut/STK11wt tumors, KRASmut/STK11mut exhibited significantly higher recurrence risk. Single-cell transcriptomics showed enhanced oxidative phosphorylation with evidence of decreased prostaglandin E2 signaling and increased IL-2 signaling in CD8+ tumor-infiltrating lymphocytes (TIL) from KRASmut/STK11mut tumors, a finding that was mirrored in KRASwt tumors that relapsed. TILs from KRASmut/STK11mut tumors expressed high levels of molecules associated with tumor residence, including CD39 and ZNF683 (HOBIT).
Conclusions: These divergent T-cell transcriptional fates suggest that T-cell maintenance and residence may be detrimental to antitumor immunity in the context of neoadjuvant ICB for resectable NSCLC, regardless of KRAS mutation status. Our work provides a basis for future investigations into the mechanisms underpinning prostaglandin E2 signaling and IL-2 signaling as they relate to T-cell immunity to cancer and to divergent clinical outcomes in KRASmut/STK11mut NSCLC treated with neoadjuvant ICB.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
S. Rosner reports grants from Conquer Cancer/American Society of Clinical Oncology during the conduct of the study, as well as personal fees from MJH, EmPartners, Axiom, ASCO Advantage, Cardinal Health, Life Sciences FGI, Regeneron, and AstraZeneca outside the submitted work. J.E. Reuss reports personal fees from AstraZeneca, Bristol Myers Squibb, Arcus, AbbVie, Daiichi Sankyo, Catalym, Seagen, Gilead, Janssen, Novocure, Regeneron, Summit Therapeutics, and Pfizer and grants from Genentech, Verastem, Nuvalent, Exelixis, and Arcus outside the submitted work. S.R. Broderick reports other support from Bristol Myers Squibb and AstraZeneca outside the submitted work. D.R. Jones reports other support from AstraZeneca and grants from Merck outside the submitted work. J.S. Deutsch reports grants from Bristol Myers Squibb outside the submitted work, as well as a patent for PCT/US2023/013869 pending. J. Chaft reports grants from NCI P30 CA008748 Cancer Center Support Grant during the conduct of the study, as well as grants and personal fees from AstraZeneca, Merck, and Genentech/Roche, grants from Bristol Myers Squibb and BeiGene, and personal fees from Guardant Health, Boehringer Ingelheim, Janssen, Eli Lilly and Company, and Sanofi-Regeneron outside the submitted work. J. Spicer reports grants, personal fees, and nonfinancial support from Bristol Myers Squibb, Merck, and AstraZeneca, grants from CLS Therapeutics, and personal fees from Roche, Daiichi Sankyo, Regeneron, Eisai, Pfizer, and Amgen outside the submitted work. J. Taube reports grants and other support from Bristol Myers Squibb, other support from AstraZeneca, Elephas, Regeneron, Merck & Co, and Lunaphore, and grants, nonfinancial support, and other support from Akoya Biosciences outside the submitted work. V. Anagnostou reports grants from AstraZeneca, Bristol Myers Squibb, Delfi Diagnostics, and Personal Genome Diagnostics and personal fees from NeoGenomics, AstraZeneca, Foundation Medicine, and Personal Genome Diagnostics outside the submitted work, as well as a patent for Cancer Genomic Analyses, ctDNA Therapeutic Response Monitoring and Immunogenomic Features of Response to Immunotherapy (63/276,525; 17/779,936; 16/312,152; 16/341,862; 17/047,006; 17/598,690) issued. J.R. Brahmer reports grants and personal fees from Bristol Myers Squibb during the conduct of the study, as well as grants and personal fees from AstraZeneca and personal fees from Summit Therapeutics and Amgen outside the submitted work. D.M. Pardoll reports grants from Bristol Myers Squibb during the conduct of the study, as well as a patent for LAG3 Blockade in Cancer Therapy PCT/US04/006133 (as well as national-stage filings, continuations, and divisional applications therefrom; JHU Ref. C04255) issued to Bristol Myers Squibb. H. Ji reports grants from NIH during the conduct of the study, as well as grants from NIH outside the submitted work. P.M. Forde reports grants from Bristol Myers Squibb during the conduct of the study as well as grants and personal fees from AstraZeneca, Bristol Myers Squibb, Novartis, Regeneron, and BioNTech and receiving consulting fees from Ascendis, AstraZeneca, Bristol Myers Squibb, CureVac, Novartis, Regeneron, G1, Genlux, Genentech, Gritstones, Merck, Janssen, F-star, Sanofi, Amgen, Fosun, Teva, Synthekine, Flame, iTeos, and Tavotek outside the submitted work. K.A. Marrone reports grants, personal fees, and nonfinancial support from Bristol Myers Squibb, personal fees and nonfinancial support from Mirati Therapeutics, and personal fees from Regeneron, Janssen, Daiichi Sankyo, Amgen, AstraZeneca, and Merus outside the submitted work. K.N. Smith reports grants from Bristol Myers Squibb during the conduct of the study; grants from AbbVie and Bristol Myers Squibb outside the submitted work; a patent for MANAFEST Technology licensed and with royalties paid from Clasp Therapeutics and a patent for TCRs Targeting Mutated Oncogenes pending; and being a Scientific Founder with stock options in Clasp Therapeutics. No disclosures were reported by the other authors.
Figures
Comment in
-
KRAS and STK11 co-mutations in resectable non-small cell lung cancer: enduring prognostic value and impaired immunotherapy response.Transl Lung Cancer Res. 2025 Jul 31;14(7):2374-2382. doi: 10.21037/tlcr-2025-463. Epub 2025 Jul 22. Transl Lung Cancer Res. 2025. PMID: 40799447 Free PMC article. No abstract available.
-
Decoding the immune landscape: KRAS and STK11 genes mutations shape CD8+ T cell response in resectable non-small-cell lung cancer after neoadjuvant therapy.Transl Lung Cancer Res. 2025 Aug 31;14(8):2918-2921. doi: 10.21037/tlcr-2025-442. Epub 2025 Aug 20. Transl Lung Cancer Res. 2025. PMID: 40948840 Free PMC article. No abstract available.
References
-
- Sanber K, Rosner S, Forde PM, Marrone KA. Neoadjuvant immunotherapy for non-small cell lung cancer. BioDrugs 2023;37:775–91. - PubMed
-
- Cottrell TR, Thompson ED, Forde PM, Stein JE, Duffield AS, Anagnostou V, et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann Oncol 2018;29:1853–60. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- LUNGevity Foundation (LUNGevity)
- P30CA008748/National Institutes of Health (NIH)
- R37CA251447/National Institutes of Health (NIH)
- P30 CA006973/CA/NCI NIH HHS/United States
- Conquer Cancer Foundation (CCF)
- P30 CA008748/CA/NCI NIH HHS/United States
- Swim Across America (SAA)
- Lung Cancer Foundation of America
- Bloomberg∼Kimmel Institute for Cancer Immunotherapy, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University (Bloomberg∼Kimmel Institute for Cancer Immunotherapy)
- American Lung Association (ALA)
- Mark Foundation For Cancer Research (The Mark Foundation for Cancer Research)
- P30CA006973/National Institutes of Health (NIH)
- R37 CA251447/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

