A PSMA-Targeted Tri-Specific Killer Engager Enhances NK Cell Cytotoxicity against Prostate Cancer
- PMID: 39545924
- PMCID: PMC11790377
- DOI: 10.1158/2326-6066.CIR-24-0273
A PSMA-Targeted Tri-Specific Killer Engager Enhances NK Cell Cytotoxicity against Prostate Cancer
Abstract
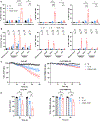
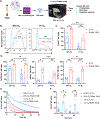
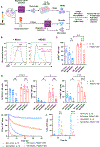
NK cell tumor infiltration is associated with good prognosis in patients with metastatic castration-resistant prostate cancer (mCRPC). NK cells recognize and kill targets by a process called natural cytotoxicity. We hypothesized that promoting an antigen-specific synapse with coactivation may enhance NK cell function in mCRPC. We describe a tri-specific killer engager (TriKE) construct that engages with the activating receptor CD16 on NK cells and prostate-specific membrane antigen (PSMA) on mCRPC cells and has an IL15 moiety that is essential for NK cell survival, proliferation, and priming. We show that the PSMA TriKE specifically binds to PSMA-expressing cells and significantly enhances expansion, degranulation, and cytokine production of NK cells derived from healthy donors or patients with prostate cancer. Bystander killing of PSMA-negative tumor cells was also achieved with PSMA TriKE treatment when cocultured with PSMA-positive cells, suggesting potential PSMA TriKE benefit in controlling tumor antigen escape. When tested under physiologic conditions recapitulating the mCRPC tumor microenvironment, NK cells treated with PSMA TriKE and prolonged exposure to hypoxia or myeloid-derived suppressor cells maintained their potent function whereas IL15-treated NK cells showed greatly impaired cytotoxicity. Finally, in vivo testing of PSMA TriKE showed improved tumor control and survival of mice as compared with IL15-treated and untreated control groups. In conclusion, PSMA TriKE demonstrates potential as a new therapy for advanced prostate cancer by providing additional signals to NK cells to maximize their antitumor potential in prostate cancer, especially in the setting of a hostile tumor microenvironment.
©2024 American Association for Cancer Research.
Figures




References
-
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin [Internet]. 2024. Jan 17;74(1):12–49. Available from: https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21820 - DOI - PubMed
-
- Scher HI, Fizazi K, Saad F, Taplin M-E, Sternberg CN, Miller K, et al. Increased Survival with Enzalutamide in Prostate Cancer after Chemotherapy. Cabot RC, Harris NL, Rosenberg ES, Shepard J-AO, Cort AM, Ebeling SH, et al., editors. N Engl J Med [Internet]. 2012. Sep 27;367(13):1187–97. Available from: http://www.nejm.org/doi/10.1056/NEJMoa1207506 - DOI - PubMed
-
- Fizazi K, Scher HI, Molina A, Logothetis CJ, Chi KN, Jones RJ, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol [Internet]. 2012. Oct;13(10):983–92. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1470204512703790 - PubMed
-
- Henríquez I, Roach M, Morgan TM, Bossi A, Gómez JA, Abuchaibe O, et al. Current and Emerging Therapies for Metastatic Castration-Resistant Prostate Cancer (mCRPC). Biomedicines [Internet]. 2021. Sep 17;9(9):1247. Available from: https://www.mdpi.com/2227-9059/9/9/1247 - PMC - PubMed
-
- Wang I, Song L, Wang BY, Rezazadeh Kalebasty A, Uchio E, Zi X. Prostate cancer immunotherapy: a review of recent advancements with novel treatment methods and efficacy. Am J Clin Exp Urol [Internet]. 2022;10(4):210–33. Available from: http://www.ncbi.nlm.nih.gov/pubmed/36051616%0Ahttp://www.pubmedcentral.n... - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

