MCRS1 sensitizes T cell-dependent immunotherapy by augmenting MHC-I expression in solid tumors
- PMID: 39545935
- PMCID: PMC11572484
- DOI: 10.1084/jem.20240959
MCRS1 sensitizes T cell-dependent immunotherapy by augmenting MHC-I expression in solid tumors
Abstract
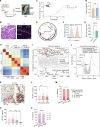
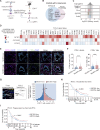
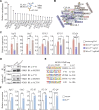
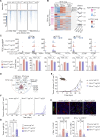
Dampened antigen presentation underscores the resistance of pancreatic cancer to T cell-mediated anti-tumor immunity, rendering immunotherapy largely ineffective. By high-throughput CRISPR activation perturbation, we discovered that the transcriptional regulator MCRS1 significantly augmented the sensitivity of mouse pancreatic cancer cells to T cell immunity in vitro and in vivo. Mechanistically, MCRS1 interacted with the transcription factor and genome organizer YY1 to coordinately increase the chromatin accessibility and expression of MHC-I genes. Elevated MCRS1 subverted MHC-I suppression and activated anti-tumor T cells, which sensitized mouse pancreatic cancer to α-PD-1 therapy. Remarkably, high MCRS1 expression was associated with increased T cell infiltration and extended survival of patients with pancreatic cancer and was predictive of favorable responses to α-PD-1 therapy in patients with lung cancer. Together, our study uncovers that MCRS1 sensitizes cancer cells to T cell immunity by transcriptionally subverting MHC-I suppression, which enhances the effectiveness of α-PD-1 therapy in mice and humans, paving the way to further improve immunotherapy against solid tumors.
© 2024 Li et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures








Similar articles
-
Glucocorticoid receptor regulates PD-L1 and MHC-I in pancreatic cancer cells to promote immune evasion and immunotherapy resistance.Nat Commun. 2021 Dec 6;12(1):7041. doi: 10.1038/s41467-021-27349-7. Nat Commun. 2021. PMID: 34873175 Free PMC article.
-
Irreversible electroporation combined with PD-L1/IL-6 dual blockade promotes anti-tumor immunity via cDC2/CD4+T cell axis in MHC-I deficient pancreatic cancer.Cancer Lett. 2025 May 1;617:217620. doi: 10.1016/j.canlet.2025.217620. Epub 2025 Mar 9. Cancer Lett. 2025. PMID: 40068706
-
Low-dose SAHA enhances CD8+ T cell-mediated antitumor immunity by boosting MHC I expression in non-small cell lung cancer.Cell Oncol (Dordr). 2025 Feb;48(1):249-264. doi: 10.1007/s13402-024-00989-9. Epub 2024 Sep 16. Cell Oncol (Dordr). 2025. PMID: 39283477 Free PMC article.
-
Recent Advances in Lung Cancer Immunotherapy: Input of T-Cell Epitopes Associated With Impaired Peptide Processing.Front Immunol. 2019 Jul 3;10:1505. doi: 10.3389/fimmu.2019.01505. eCollection 2019. Front Immunol. 2019. PMID: 31333652 Free PMC article. Review.
-
Cancer immune escape: MHC expression in primary tumours versus metastases.Immunology. 2019 Dec;158(4):255-266. doi: 10.1111/imm.13114. Epub 2019 Oct 1. Immunology. 2019. PMID: 31509607 Free PMC article. Review.
Cited by
-
Decoding MHC loss: Molecular mechanisms and implications for immune resistance in cancer.Clin Transl Med. 2025 Jul;15(7):e70403. doi: 10.1002/ctm2.70403. Clin Transl Med. 2025. PMID: 40673641 Free PMC article. Review.
-
Global research prospects and trends in TFH cells and tumors: a bibliometric analysis.Front Oncol. 2025 Feb 14;15:1443890. doi: 10.3389/fonc.2025.1443890. eCollection 2025. Front Oncol. 2025. PMID: 40027134 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

