Longitudinal evaluation of hemodynamic blood and echocardiographic biomarkers for the prediction of BPD and BPD-related pulmonary hypertension in very-low-birth-weight preterm infants
- PMID: 39546006
- PMCID: PMC11567987
- DOI: 10.1007/s00431-024-05841-8
Longitudinal evaluation of hemodynamic blood and echocardiographic biomarkers for the prediction of BPD and BPD-related pulmonary hypertension in very-low-birth-weight preterm infants
Abstract
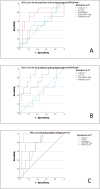
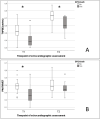
Very-low-birth-weight infants (VLBW, < 1500 g) are at risk of developing bronchopulmonary dysplasia (BPD) and are at risk for BPD-related pulmonary hypertension (PH). The longitudinal measurement of innovative blood and echocardiographic biomarkers might allow for a risk stratification of these infants. A prospective single-center cohort study was conducted between 01/2021 and 06/2023. Inclusion criteria were the combination of a birth weight < 1500 g and a gestational age (GA) ≤ 30/0 weeks. Assessment timepoints: T1 (day 7), T2 (day 28), and T3 (at 36 weeks post-menstrual age, PMA). Overall, 71 preterm infants were included for final analysis. The Zlog-transformed NTproBNPZlog (at T1 AUC 0.772; p = 0.019; at T2 AUC 0.874, p = 0.002), and endothelin-1 (ET1, at T1 AUC 0.789, p = 0.013) were identified as an early predictive biomarker for BPD/death in the univariate analysis. Additionally, echocardiographic markers of ventricular function and PH at T1 were predictive for BPD/death in the univariate analysis, with the highest predictivity found for the tricuspid annular plane systolic excursion-TAPSE (AUC 0.748, p = 0.016) and the pulmonary artery acceleration time to right ventricular ejection time (PAAT/RVET; AUC 0.744, p = 0.043). Regarding predictability of mortality alone NTroBNPZlog (at T1 AUC 0.973, p = 0.000), and CA125 (at T1 AUC 0.747, p = 0.008) were identified as potential predictors, as well as TAPSE (AUC 0.926, p = 0.000), and PAAT/RVET (AUC 0.985, p = 0.000) Several biomarkers including ET-1 (at T1 AUC 0.893, p = 0.000), TAPSE (AUC 0.974, p = 0.000), and PAAT/RVET (AUC 1.0, p = 0.000) at T1 were identified as univariate predictors for BPD-PH. In the multivariate analysis, no biomarker was identified as an independent predictor of the primary endpoint.
Conclusion: Mainly at an early stage of postnatal neonatal care in VLBW preterm infants, several biomarkers were found to be associated with the combined endpoint BPD/death and BPD-PH. New candidates of blood biomarkers (NTproBNPZlog, ET-1, and CA125) and echocardiographic markers (TAPSE, PAAT/RVET) might serve as innovative predictors for BPD, BPD-PH, and adverse outcomes in VBLW infants.
What is known: • VLBW infants are at risk for the development of BPD and BPD-related PH, which both are main contributors for short and long-term morbidity and mortality. Several studies in the past focused on the evaluation of circulating blood biomarkers and biomarkers from echocardiographic assessment of these infants. But to date, there is still a lack on longitudinal prospective studies especially in VLBW infants.
What is new: • For the first time, this set of selected blood biomarkers (with the first description of Zlog-transformed NTproBNP and CA125 in preterm infants) and several echocardiographic markers were analyzed in a prospective longitudinal study from birth until 36 weeks post menstrual age in VLBW infants. Our data help clinicians to identify preterm infants at risk for BPD, BPD-PH and death and to offer new candidates of biomarkers. This might help to facilitate decision making and guidance of therapy in these highly vulnerable patients.
Keywords: Biomarker; Bronchopulmonary dysplasia; Echocardiography; Mortality; Preterm infants; Pulmonary hypertension.
© 2024. The Author(s).
Conflict of interest statement
Figures

References
-
- Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, Kirpalani H, Laughon MM, Poindexter BB, Duncan AF, Yoder BA, Eichenwald EC, DeMauro SB (2019) The diagnosis of bronchopulmonary dysplasia in very preterm infants An evidence-based approach. Am J Respir Crit Care Med 200(6):751–759. 10.1164/rccm.201812-2348OC - PMC - PubMed
-
- Siffel C, Kistler KD, Lewis JFM, Sarda SP (2021) Global incidence of bronchopulmonary dysplasia among extremely preterm infants: a systematic literature review. J Matern Fetal Neonatal Med 34(11):1721–1731. 10.1080/14767058.2019.1646240 - PubMed
-
- Moreira A, Noronha M, Joy J, Bierwirth N, Tarriela A, Naqvi A, Zoretic S, Jones M, Marotta A, Valadie T, Brick J, Winter C, Porter M, Decker I, Bruschettini M, Ahuja SK (2024) Rates of bronchopulmonary dysplasia in very low birth weight neonates: a systematic review and meta-analysis. Respir Res 25(1):219. 10.1186/s12931-024-02850-x - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

