Whole-body vibration elicits 40 Hz cortical gamma oscillations and ameliorates age-related cognitive impairment through hippocampal astrocyte synapses in male rats
- PMID: 39546054
- PMCID: PMC11568021
- DOI: 10.1007/s10522-024-10154-2
Whole-body vibration elicits 40 Hz cortical gamma oscillations and ameliorates age-related cognitive impairment through hippocampal astrocyte synapses in male rats
Abstract
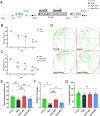
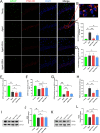
Age-related cognitive impairment is a prevalent issue in developed societies. Gamma oscil2lations at 40 Hz have been identified as a potential therapeutic approach for age-related cognitive decline and can be induced through various modalities, including auditory, visual, electrical, and magnetic stimulation. In this study, we investigated a novel modality of stimulation: whole-body vibration at 40 Hz. We examined the effects of 40 Hz vibration on cognitive performance and associated neuronal activity in the brains of aged male rats. Our findings revealed that only vibration at 40 Hz, rather than 20 Hz or 80 Hz, elicited cortical gamma oscillations in aged male rats. Additionally, following 8 weeks of prolonged treatment, the implementation of 40 Hz whole-body vibration significantly augmented the cognitive function of aged male rats as evidenced by behavioral assessments. Mechanistic studies demonstrated that these beneficial effects were attributed to the reduction of neuronal apoptosis in hippocampal CA1 through regulation of synaptic connections between astrocytes and neurons via 40 Hz gamma oscillations. Collectively, this suggests a promising intervention for age-related cognitive decline and identifies neuron-astrocyte synapses as potential therapeutic targets.
Keywords: Cognitive dysfunction; Gamma oscillations; Neuron-astrocyte synapses; Vibration.
© 2024. The Author(s).
Conflict of interest statement
Figures



References
-
- Adamsky A, Kol A, Kreisel T et al (2018) Astrocytic activation generates de novo neuronal potentiation and memory enhancement. Cell 174:59-71.e14. 10.1016/j.cell.2018.05.002 - PubMed
-
- Anderson ND (2019) State of the science on mild cognitive impairment (MCI). CNS Spectr 24:78–87. 10.1017/S1092852918001347 - PubMed
-
- Başar E, Başar-Eroğlu C, Güntekin B, Yener GG (2013) Brain’s alpha, beta, gamma, delta, and theta oscillations in neuropsychiatric diseases: proposal for biomarker strategies. Suppl Clin Neurophysiol 62:19–54. 10.1016/b978-0-7020-5307-8.00002-8 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

