Racial and Ethnic Differences in Prostate Cancer Epidemiology Across Disease States in the VA
- PMID: 39546308
- PMCID: PMC11568464
- DOI: 10.1001/jamanetworkopen.2024.45505
Racial and Ethnic Differences in Prostate Cancer Epidemiology Across Disease States in the VA
Abstract
Importance: Prostate cancer (PC) care has evolved rapidly as a result of changes in prostate-specific antigen testing, novel imaging, and newer treatments. The impact of these changes on PC epidemiology and racial disparities across disease states remains underexplored.
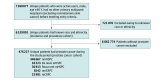
Objective: To characterize racial and ethnic differences in the epidemiology of PC states, including nonmetastatic hormone-sensitive PC (nmHSPC), metastatic HSPC (mHSPC), nonmetastatic castration-resistant PC (nmCRPC), and metastatic CRPC (mCRPC).
Design, setting, and participants: This is a retrospective, population-based cohort study of male US veterans aged 40 years and older with known race and ethnicity and no non-PC malignant neoplasm before study entry receiving care through the Veterans Health Administration. The study period was from 2012 to 2020, with follow-up through 2021. To identify active users, data capture included visits 18 months before and after the study period. Data analysis was performed from March to August 2023.
Exposure: Self-identified race and ethnicity, classified as Black, White, or Hispanic.
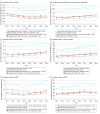
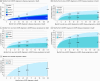
Main outcomes and measures: The primary outcomes were annual age-adjusted incidence rates (IRs) and point prevalence for PC states by race and ethnicity. Trends were evaluated using joinpoint regression. Time to disease progression or death was estimated using nonparametric cumulative incidence. Competing risk models adjusted for age assessed the association of race and ethnicity on disease progression.
Results: The study included 6 539 001 veterans (median [IQR] age, 65 [56-74] years), of whom 476 227 had PC (median [IQR] age, 69 [63-75] years). IRs varied by time frame and disease state. Across all states and years, the relative risk among Black vs White patients ranged from 2.09 (95% CI, 2.01-2.18; P < .001) for nmHSPC in 2012 to 4.12 (95% CI, 3.39-5.02; P < .001) for nmCRPC in 2017. In nmHSPC, hazard ratios for progression to mHSPC and nmCRPC were 1.36 (95% CI, 1.33-1.40) and 1.60 (95% CI, 1.51-1.70), respectively, for Black patients and 1.38 (95% CI, 1.31-1.45) and 1.55 (95% CI, 1.40-1.72), respectively, for Hispanic patients vs White patients. In contrast, in mCRPC, the hazard ratio for death was lower for Black (0.84; 95% CI, 0.81-0.88) and Hispanic (0.76; 95% CI, 0.69-0.83) patients compared with White patients.
Conclusions and relevance: This cohort study of veterans found that Black patients had more than 2-fold higher incidence of all disease states vs White patients. Progression risk was higher for Black and Hispanic patients in early-stage disease, but lower in later disease stages. Despite equal access, Black patients disproportionately experience PC, although progression risks relative to White patients differed according to disease state.
Conflict of interest statement
Figures
Comment in
- doi: 10.1001/jamanetworkopen.2024.45522
Similar articles
-
Somatic Tumor Next-Generation Sequencing in US Veterans With Metastatic Prostate Cancer.JAMA Netw Open. 2025 May 1;8(5):e259119. doi: 10.1001/jamanetworkopen.2025.9119. JAMA Netw Open. 2025. PMID: 40354055 Free PMC article.
-
Racial and Ethnic Disparities in Receipt of ERBB2-Targeted Therapy for Breast Cancer, 2010-2020.JAMA Netw Open. 2025 May 1;8(5):e258086. doi: 10.1001/jamanetworkopen.2025.8086. JAMA Netw Open. 2025. PMID: 40310643 Free PMC article.
-
Association of Race and Ethnicity With Initial Prescription of Antiretroviral Therapy Among People With HIV in the US.JAMA. 2023 Jan 3;329(1):52-62. doi: 10.1001/jama.2022.23617. JAMA. 2023. PMID: 36594946 Free PMC article.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
-
The impact of race on survival in metastatic prostate cancer: a systematic literature review.Prostate Cancer Prostatic Dis. 2023 Sep;26(3):461-474. doi: 10.1038/s41391-023-00710-1. Epub 2023 Aug 17. Prostate Cancer Prostatic Dis. 2023. PMID: 37592001 Free PMC article.
Cited by
-
Disparities Between Rural and Urban Patients With Prostate Cancer in Nebraska.Cancer Med. 2025 Mar;14(6):e70812. doi: 10.1002/cam4.70812. Cancer Med. 2025. PMID: 40125616 Free PMC article.
-
Secondary Analysis of PSA and BCR-Free Survival in Asian Prostate Cancer Patients.Cancer Manag Res. 2025 Jun 24;17:1205-1214. doi: 10.2147/CMAR.S527092. eCollection 2025. Cancer Manag Res. 2025. PMID: 40584226 Free PMC article.
-
Somatic Tumor Next-Generation Sequencing in US Veterans With Metastatic Prostate Cancer.JAMA Netw Open. 2025 May 1;8(5):e259119. doi: 10.1001/jamanetworkopen.2025.9119. JAMA Netw Open. 2025. PMID: 40354055 Free PMC article.
References
-
- Hofman MS, Lawrentschuk N, Francis RJ, et al. ; proPSMA Study Group Collaborators . Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395(10231):1208-1216. doi:10.1016/S0140-6736(20)30314-7 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

