LincROR promotes tumor growth of colorectal cancer through the miR-145/WNT2B/WNT10A/Wnt/β-catenin regulatory axis
- PMID: 39546475
- PMCID: PMC11567539
- DOI: 10.1371/journal.pone.0312417
LincROR promotes tumor growth of colorectal cancer through the miR-145/WNT2B/WNT10A/Wnt/β-catenin regulatory axis
Abstract
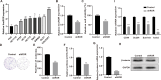
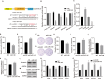
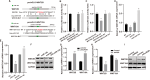
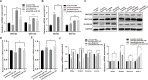
Colorectal cancer (CRC) is a prevalent form of malignant tumor, and the current clinical treatments are far from satisfactory. Identifying new therapeutic targets is therefore essential for clinical practices. The long intergenic non-protein coding RNA lincROR has been shown to play a significant role in the tumorigenesis of various cancers. However, the molecular mechanism underlying lincROR-mediated CRC tumorigenesis remains unclear. In the present study, we found that knockdown of lincROR significantly inhibited cell viability in vitro, while its overexpression promoted tumor growth in vivo. Mechanistically, lincROR acted as a miRNA sponge for miR-145, thereby elevating the expression of the target genes WNT2B and WNT10A. The overexpression of WNT2B and WNT10A definitely activated the Wnt/β-catenin pathway, thus led to promoting tumorigenesis in CRC. In summary, our findings identified lincROR as a novel activator of the Wnt/β-catenin pathway by serving as a miRNA sponge for miR-145 and facilitating tumorigenesis, which suggests that lincROR may be a potential therapeutic target for CRC patients.
Copyright: © 2024 Deng et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
All authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Mi Y, Li Y, He Z, Chen D, Hong Q, You J. Upregulation of Linc-ROR Promotes the Proliferation, Migration, and Invasion of Gastric Cancer Cells Through miR-212-3p/FGF7 Axis. Cancer Manag Res. 2021;13:899–912. Epub 2021/02/11. doi: 10.2147/CMAR.S287775 ; PubMed Central PMCID: PMC7867499. - DOI - PMC - PubMed
-
- Shao J, Shi CJ, Li Y, Zhang FW, Pan FF, Fu WM, et al. LincROR Mediates the Suppressive Effects of Curcumin on Hepatocellular Carcinoma Through Inactivating Wnt/beta-Catenin Signaling. Front Pharmacol. 2020;11:847. Epub 2020/07/28. doi: 10.3389/fphar.2020.00847 ; PubMed Central PMCID: PMC7351502. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

