Salmonella virulence factors induce amino acid malabsorption in the ileum to promote ecosystem invasion of the large intestine
- PMID: 39546570
- PMCID: PMC11588050
- DOI: 10.1073/pnas.2417232121
Salmonella virulence factors induce amino acid malabsorption in the ileum to promote ecosystem invasion of the large intestine
Abstract
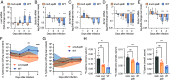
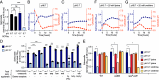
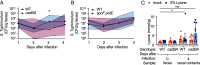
The gut microbiota produces high concentrations of antimicrobial short-chain fatty acids (SCFAs) that restrict the growth of invading microorganisms. The enteric pathogen Salmonella enterica serovar (S.) Typhimurium triggers inflammation in the large intestine to ultimately reduce microbiota density and bloom, but it is unclear how the pathogen gains a foothold in the homeostatic gut when SCFA-producing commensals are abundant. Here, we show that S. Typhimurium invasion of the ileal mucosa triggers malabsorption of dietary amino acids to produce downstream changes in nutrient availability in the large intestine. In gnotobiotic mice engrafted with a community of 17 human Clostridia isolates, S. Typhimurium virulence factors triggered marked changes in the cecal metabolome, including an elevated abundance of amino acids. In an ex vivo fecal culture model, we found that two of these amino acids, lysine and ornithine, countered SCFA-mediated growth inhibition by restoring S. Typhimurium pH homeostasis through the inducible amino acid decarboxylases CadA and SpeF, respectively. In a mouse model of gastrointestinal infection, S. Typhimurium CadA activity depleted dietary lysine to promote cecal ecosystem invasion in the presence of an intact microbiota. From these findings, we conclude that virulence factor-induced malabsorption of dietary amino acids in the small intestine changes the nutritional environment of the large intestine to provide S. Typhimurium with resources needed to counter growth inhibition by microbiota-derived SCFAs.
Keywords: Salmonella; colonization resistance; microbiota; short-chain fatty acids.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

References
-
- Salmond C. V., Kroll R. G., Booth I. R., The effect of food preservatives on pH homeostasis in Escherichia coli. J. Gen. Microbiol. 130, 2845–2850 (1984). - PubMed

