B cell adapter for PI 3-kinase (BCAP) coordinates antigen internalization and trafficking through the B cell receptor
- PMID: 39546610
- PMCID: PMC11566990
- DOI: 10.1126/sciadv.adp1747
B cell adapter for PI 3-kinase (BCAP) coordinates antigen internalization and trafficking through the B cell receptor
Abstract
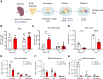
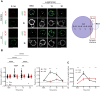
B cell adapter for PI 3-kinase (BCAP) is an adaptor molecule associated with signaling through multiple immune receptors, including the B cell receptor (BCR). However, B cell-intrinsic role of BCAP in antibody responses is unclear. We investigated the role of BCAP in B cell response to viral particles and found a previously unidentified mechanism by which BCAP regulates antigen-specific responses. B cell-specific deletion of BCAP in mice leads to decreases in antigen-specific responses through defects in BCR-antigen endocytosis. BCAP is necessary to orchestrate actin reorganization around the antigen for efficient endocytosis through BCR and intracellular processing of antigens. Therefore, loss of BCAP from B cells leads to defects in antigen endocytosis, hampering the propagation of antigen-derived signals and decreasing the ability of B cells to present antigens to T cells. Thus, our study clarifies how BCAP regulates B cell responses to complex antigens and elucidates that antigen positioning inside B cells determines different B cell activation outcomes.
Figures





References
-
- Okada T., Maeda A., Iwamatsu A., Gotoh K., Kurosaki T., BCAP: The tyrosine kinase substrate that connects B cell receptor to phosphoinositide 3-kinase activation. Immunity 13, 817–827 (2000). - PubMed
-
- Yamazaki T., Kurosaki T., Contribution of BCAP to maintenance of mature B cells through c-Rel. Nat. Immunol. 4, 780–786 (2003). - PubMed
-
- Aiba Y., Kameyama M., Yamazaki T., Tedder T. F., Kurosaki T., Regulation of B-cell development by BCAP and CD19 through their binding to phosphoinositide 3-kinase. Blood 111, 1497–1503 (2008). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

