A real-world comparison of commercial-use axicabtagene ciloleucel and lisocabtagene maraleucel in large B-cell lymphoma
- PMID: 39546746
- PMCID: PMC11808612
- DOI: 10.1182/bloodadvances.2024012992
A real-world comparison of commercial-use axicabtagene ciloleucel and lisocabtagene maraleucel in large B-cell lymphoma
Abstract
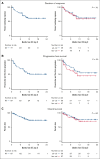
Lisocabtagene maraleucel (liso-cel) and axicabtagene ciloleucel (axi-cel) are anti-CD19 chimeric antigen receptor (CAR) T-cell therapies approved for relapsed and refractory large B-cell lymphoma (LBCL); however, there is currently no published data on liso-cel outside of clinical trials nor any data comparing these therapies. In this retrospective analysis, we reviewed patients with LBCL receiving liso-cel or axi-cel at a single institution in the third-line setting. From June 2021 to September 2022, a total of 50 patients received axi-cel and 37 liso-cel. Baseline patient characteristics were similar, aside from older age in liso-cel recipients. The median time from leukapheresis to CAR T-cell infusion was significantly longer for liso-cel (41 days) than axi-cel (30 days). Complete response rates were not significantly different between axi-cel (72%) and liso-cel (62%). At a median follow-up of 11 months, progression-free survival (PFS) was not significantly different between axi-cel and liso-cel cohorts, with 12-month PFS of 59% and 44%, respectively. However, on a propensity score analysis, an inferior PFS was observed with liso-cel (hazard ratio, 2.95; 95% confidence interval , 1.14-7.60). The rates of cytokine release syndrome, immune effector cell-associated neurotoxicity syndrome, and prolonged neutropenia were higher with axi-cel than liso-cel. Overall, direct comparison of axi-cel and liso-cel cohorts shows similar key outcomes including response rate and PFS, but prolonged wait times for liso-cel may have resulted in biased selection of patients with more favorable characteristics for liso-cel. When accounting for these higher-risk characteristics, an inferior PFS is observed with liso-cel compared with axi-cel. These findings warrant further evaluation in a multicenter setting.
© 2025 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: D.Q. reports advisory board fees from Genmab and ADC Therapeutics. P.A. reports consulting roles for Merck, Bristol Myers Squibb (BMS), Pfizer, Affimed, Adaptive, Infinity, ADC Therapeutics, Celgene, MorphoSys, Daiichi Sankyo, Miltenyi, Tessa, Genmab, C4, Enterome, Regeneron, Epizyme, AstraZeneca, Genentech/Roche, Xencor, Foresight, and ATB Therapeutics; research funding from Kite, Merck, BMS, Affimed, Adaptive, Tensha, Otsuka, Sigma Tau, Genentech/Roche, IGM Biosciences, and AstraZeneca; and honoraria from Merck and BMS. J.L.C. reports consulting fees from ADC Therapeutics, Seagen, Kite, Incite/MorphoSys, and Regeneron, and research funding from Genentech/Roche, Merck, AbbVie, and Bayer. E.D.J. reports consulting fees from Syros and Takeda, and research funding from Acerta, Janssen, Novartis, and Pharmacyclics. A.S.L. reports advisory board fees from Kite and Seagen; consulting fees from Research to Practice, Genmab, Adaptive Biotechnologies, BMS, AbbVie, Intellia, and Epizyme; and research funding from BMS, Merck, Genentech/Roche, and Genmab. C.A.J. served as a consultant for Kite/Gilead, Novartis, BMS/Celgene, Abintus Bio, ImmPACT Bio, Caribou Bio, MorphoSys, ADC Therapeutics, AbbVie, AstraZeneca, Ipsen, Sana, Synthekine, Daiichi Sankyo, and Janssen, and has received research funding from Kite/Gilead and Pfizer. The remaining authors declare no competing financial interests.
Figures


References
-
- Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. - PubMed
-
- Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640–654. - PubMed
-
- Kamdar M, Solomon SR, Arnason J, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399(10343):2294–2308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

