Netrin1 patterns the dorsal spinal cord through modulation of Bmp signaling
- PMID: 39547237
- PMCID: PMC11756817
- DOI: 10.1016/j.celrep.2024.114954
Netrin1 patterns the dorsal spinal cord through modulation of Bmp signaling
Abstract
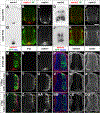
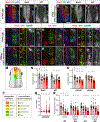
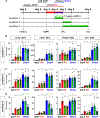
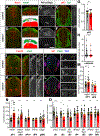
We have identified an unexpected role for netrin1, a canonical axonal guidance cue, as a suppressor of bone morphogenetic protein (Bmp) signaling in the developing dorsal spinal cord. Using a combination of gain- and loss-of-function approaches in chicken and mouse embryonic models, as well as mouse embryonic stem cells (mESCs), we have observed that manipulating the level of netrin1 specifically alters the patterning of the Bmp-dependent dorsal interneurons (dIs), dI1-dI3. Altered netrin1 levels also change Bmp signaling activity, as assessed using bioinformatic approaches, as well as monitoring phosophoSmad1/5/8 activation, the canonical intermediate of Bmp signaling, and Id levels, a known Bmp target. Together, these studies support the hypothesis that netrin1 acts from the intermediate spinal cord to regionally confine Bmp signaling to the dorsal spinal cord. Thus, netrin1 has reiterative activities shaping dorsal spinal circuits, first by regulating cell fate decisions and then acting as a guidance cue to direct axon extension.
Keywords: Bmp; Bmp inhibition; CP: Developmental biology; CP: Neuroscience; cell fate; dorsal interneurons; interneuron differentiation; netrin1; neural development; patterning; spinal cord.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


Update of
-
Netrin1 patterns the dorsal spinal cord through modulation of Bmp signaling.bioRxiv [Preprint]. 2023 Nov 4:2023.11.02.565384. doi: 10.1101/2023.11.02.565384. bioRxiv. 2023. Update in: Cell Rep. 2024 Nov 26;43(11):114954. doi: 10.1016/j.celrep.2024.114954. PMID: 37961605 Free PMC article. Updated. Preprint.
Similar articles
-
Netrin1 patterns the dorsal spinal cord through modulation of Bmp signaling.bioRxiv [Preprint]. 2023 Nov 4:2023.11.02.565384. doi: 10.1101/2023.11.02.565384. bioRxiv. 2023. Update in: Cell Rep. 2024 Nov 26;43(11):114954. doi: 10.1016/j.celrep.2024.114954. PMID: 37961605 Free PMC article. Updated. Preprint.
-
Netrin1/DCC signaling promotes neuronal migration in the dorsal spinal cord.Neural Dev. 2016 Oct 26;11(1):19. doi: 10.1186/s13064-016-0074-x. Neural Dev. 2016. PMID: 27784329 Free PMC article.
-
Signaling through BMP type 1 receptors is required for development of interneuron cell types in the dorsal spinal cord.Development. 2004 Nov;131(21):5393-403. doi: 10.1242/dev.01379. Epub 2004 Oct 6. Development. 2004. PMID: 15469980
-
New perspectives on the mechanisms establishing the dorsal-ventral axis of the spinal cord.Curr Top Dev Biol. 2019;132:417-450. doi: 10.1016/bs.ctdb.2018.12.010. Epub 2018 Dec 26. Curr Top Dev Biol. 2019. PMID: 30797516 Free PMC article. Review.
-
Dorsal commissural axon guidance in the developing spinal cord.Curr Top Dev Biol. 2021;142:197-231. doi: 10.1016/bs.ctdb.2020.10.009. Epub 2020 Nov 19. Curr Top Dev Biol. 2021. PMID: 33706918 Free PMC article. Review.
References
-
- Kennedy TE, Serafini T, de la Torre JR, and Tessier-Lavigne M. (1994). Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell 78, 425–435. - PubMed
-
- Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, and Tessier-Lavigne M. (1994). The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell 78, 409–424. - PubMed
-
- Deiner MS, Kennedy TE, Fazeli A, Serafini T, Tessier-Lavigne M, and Sretavan DW (1997). Netrin-1 and DCC mediate axon guidance locally at the optic disc: loss of function leads to optic nerve hypoplasia. Neuron 19, 575–589. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

