Performance of stool-based molecular tests and processing methods for paediatric tuberculosis diagnosis: a systematic review and meta-analysis
- PMID: 39547244
- PMCID: PMC12062341
- DOI: 10.1016/j.lanmic.2024.100963
Performance of stool-based molecular tests and processing methods for paediatric tuberculosis diagnosis: a systematic review and meta-analysis
Abstract
Background: There has been a global pursuit to improve the diagnosis of tuberculosis in young children by applying diagnostic methods on accessible biospecimens such as stool. We aimed to conduct a systematic review on the accuracy of stool-based molecular tests for tuberculosis diagnosis in children and to assess the impact of the available pre-processing methods and other design characteristics.
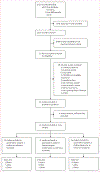
Methods: In this systematic review and meta-analysis, we evaluated studies in children younger than 16 years with presumptive tuberculosis that were published in English, Spanish, French, and Portuguese from Jan 1, 2000, to May 3, 2024, in MEDLINE, Embase, and Embase Classic, comparing the molecular detection of Mycobacterium tuberculosis DNA in stool with microbiological tests on other samples or a clinical diagnosis. We did not exclude studies based on geographical location, sample size, or study design if they were reporting primary data. Two independent reviewers (LC-C and SM) screened titles, abstracts, and full-text articles for eligibility and extracted data on study characteristics, study population, and diagnostic performance. If information relevant to the main analysis was not reported in the article, the corresponding authors were contacted. Point estimates and 95% CIs were calculated for sensitivity and specificity for each study and for the different molecular tests (Xpert MTB/RIF, Xpert Ultra MTB/RIF [Cepheid, Sunnyvale, CA, USA], and other tests) versus a reference standard (culture only, any bacteriological confirmation, and tuberculosis case definition). Sensitivity and specificity were stratified by the stool processing method. We also quantified the additionality of stool Xpert Ultra tests for tuberculosis bacteriological confirmation. The protocol was registered with PROSPERO, CRD42022341514.
Findings: A total of 4521 records were identified through the database search, one record was identified from an article bibliography, and 67 studies were retained for full-text reading. 39 studies were included in the qualitative synthesis, 35 of which were included in the meta-analyses. When using any bacteriological confirmation from a respiratory sample as the reference standard, stool Xpert sensitivity was 0·60 (95% CI 0·48-0·71), stool Xpert Ultra sensitivity was 0·73 (0·63-0·81), and sensitivity was 0·44 (0·29-0·60) for other in-house molecular methods combined. When using tuberculosis case definition as the reference standard, stool Xpert sensitivity was 0·23 (0·11-0·41), stool Xpert Ultra sensitivity was 0·38 (0·22-0·56), and sensitivity was 0·17 (0·09-0·23) for other in-house molecular methods. The addition of stool Xpert Ultra increased bacteriological confirmation of tuberculosis by 38·6% overall. Further, the utilisation of centrifuge-free simplified methods improved the sensitivity of stool Xpert Ultra when using any bacteriological confirmation as a reference standard (0·77 [0·66-0·85] for centrifuge-free methods vs 0·61 [0·41-0·78] for non-centrifuge-free methods).
Interpretation: This systematic review and meta-analysis supports the use of Xpert Ultra in stool samples as a diagnostic tool for paediatric tuberculosis diagnosis. Stool-based Xpert Ultra can contribute to increase the bacteriological confirmation in this population, even when respiratory specimens are also tested.
Funding: The EDCTP2 programme supported by the EU via Stool4TB Project and the European Society of Pediatric Infectious Diseases.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests MB's institution received UNITAID funding for the conduct of one of the studies included in the systematic review (Marcy et al 2022(60)). All other authors declare no competing interests.
Figures



References
-
- WHO. Global tuberculosis report. 2024. https://iris.who.int/bitstream/handle/10665/379339/9789240101531-eng.pdf... (accessed Nov 6, 2024).
-
- Marais BJ, Gie RP, Hesseling AC, et al. A refined symptom-based approach to diagnose pulmonary tuberculosis in children. Pediatrics 2006; 118: e1350–59. - PubMed
-
- Starke JR. Pediatric tuberculosis: time for a new approach. Tuberculosis (Edinb) 2003; 83: 208–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

