Antiseizure medication use during pregnancy and children's neurodevelopmental outcomes
- PMID: 39548057
- PMCID: PMC11568279
- DOI: 10.1038/s41467-024-53813-1
Antiseizure medication use during pregnancy and children's neurodevelopmental outcomes
Abstract
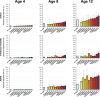
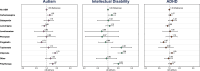
The teratogenic potential of valproate in pregnancy is well established; however, evidence regarding the long-term safety of other antiseizure medications (ASMs) during pregnancy remains limited. Using routinely collected primary care data from the UK and nationwide Swedish registries to create a cohort of 3,182,773 children, of which 17,495 were exposed to ASMs in pregnancy, we show that those exposed to valproate were more likely to receive a diagnosis of autism, intellectual disability, and ADHD, when compared to children not exposed to ASMs. Additionally, children exposed to topiramate were 2.5 times more likely to be diagnosed with intellectual disability (95% CI: 1.23-4.98), and those exposed to carbamazepine were 1.25 times more likely to be diagnosed with autism (95% CI: 1.05-1.48) and 1.30 times more likely to be diagnosed with intellectual disability (95% CI: 1.01-1.69). There was little evidence that children exposed to lamotrigine in pregnancy were more likely to receive neurodevelopmental diagnoses. While further research is needed, these findings may support considering safer treatment alternatives well before conception when clinically appropriate.
© 2024. The Author(s).
Conflict of interest statement
Figures

References
-
- Medicines & Healthcare products Regulatory Agency (MHRA). Guidance: valproate use by women and girls: UK Government. https://www.gov.uk/guidance/valproate-use-by-women-and-girls (2018).
-
- Sisodiya, S. M., Epilepsy Advisory Group for the Association of British Neurologists.Valproate and childbearing potential: new regulations. Pract. Neurol.18, 176–178 (2018). - PubMed
-
- Maimburg, R. D. & Vaeth, M. Perinatal risk factors and infantile autism. Acta Psychiatr. Scand.114, 257–264 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

