VSV∆M51 drives CD8+ T cell-mediated tumour regression through infection of both cancer and non-cancer cells
- PMID: 39548070
- PMCID: PMC11567966
- DOI: 10.1038/s41467-024-54111-6
VSV∆M51 drives CD8+ T cell-mediated tumour regression through infection of both cancer and non-cancer cells
Abstract
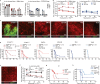
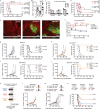
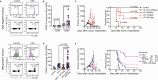
Oncolytic viruses (OV) are designed to selectively infect and kill cancer cells, while simultaneously eliciting antitumour immunity. The mechanism is expected to originate from infected cancer cells. However, recent reports of tumour regression unaccompanied by cancer cell infection suggest a more complex mechanism of action. Here, we engineered vesicular stomatitis virus (VSV)ΔM51-sensitive and VSVΔM51-resistant tumour lines to elucidate the role of OV-infected cancer and non-cancer cells. We found that, while cancer cell infections elicit oncolysis and antitumour immunity as expected, infection of non-cancer cells alone can also contribute to tumour regression. This effect is partly attributed to the systemic production of cytokines that promote dendritic cell (DC) activation, migration and antigen cross-presentation, leading to magnified antitumour CD8+ T cell activation and tumour regression. Such OV-induced antitumour immunity is complementary to PD-1 blockade. Overall, our results reveal mechanistic insights into OV-induced antitumour immunity that can be leveraged to improve OV-based therapeutics.
© 2024. The Author(s).
Conflict of interest statement
Figures





References
-
- Lichty, B. D., Breitbach, C. J., Stojdl, D. F. & Bell, J. C. Going viral with cancer immunotherapy. Nat. Rev. Cancer14, 559–567 (2014). - PubMed
-
- Liang, M. Oncorine, the world first oncolytic virus medicine and its update in China. Curr. Cancer Drug Targets18, 171–176 (2018). - PubMed
-
- Andtbacka, R. H. I. et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol.33, 2780–2788 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 420828/Gouvernement du Canada | Canadian Institutes of Health Research (Instituts de Recherche en Santé du Canada)
- 156035/Gouvernement du Canada | Canadian Institutes of Health Research (Instituts de Recherche en Santé du Canada)
- ACHF21-0845/Alberta Children's Hospital Foundation
- ACTION-20/Canadian Cancer Society Research Institute (Société Canadienne du Cancer)
- 75N92020D00005/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

