A single NLR gene confers resistance to leaf and stripe rust in wheat
- PMID: 39548072
- PMCID: PMC11568145
- DOI: 10.1038/s41467-024-54068-6
A single NLR gene confers resistance to leaf and stripe rust in wheat
Abstract
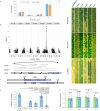
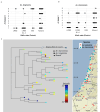
Nucleotide-binding leucine-rich repeat (NLR) disease resistance genes typically confer resistance against races of a single pathogen. Here, we report that Yr87/Lr85, an NLR gene from Aegilops sharonensis and Aegilops longissima, confers resistance against both P. striiformis tritici (Pst) and Puccinia triticina (Pt) that cause stripe and leaf rust, respectively. Yr87/Lr85 confers resistance against Pst and Pt in wheat introgression as well as transgenic lines. Comparative analysis of Yr87/Lr85 and the cloned Triticeae NLR disease resistance genes shows that Yr87/Lr85 contains two distinct LRR domains and that the gene is only found in Ae. sharonensis and Ae. longissima. Allele mining and phylogenetic analysis indicate multiple events of Yr87/Lr85 gene flow between the two species and presence/absence variation explaining the majority of resistance to wheat leaf rust in both species. The confinement of Yr87/Lr85 to Ae. sharonensis and Ae. longissima and the resistance in wheat against Pst and Pt highlight the potential of these species as valuable sources of disease resistance genes for wheat improvement.
© 2024. The Author(s).
Conflict of interest statement
Figures


References
-
- Khan, M. H., Bukhari, A., Dar, Z. A. & Rizvi, S. M. Status and strategies in breeding for rust resistance in wheat. Agric. Sci.04, 292–301 (2013).
-
- Savary, S. et al. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol.3, 430–439 (2019). - PubMed
-
- Dinh, H. X., Singh, D., Periyannan, S., Park, R. F. & Pourkheirandish, M. Molecular genetics of leaf rust resistance in wheat and barley. Theor. Appl Genet.133, 2035–2050 (2020). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

