Urease-powered nanomotor containing STING agonist for bladder cancer immunotherapy
- PMID: 39548120
- PMCID: PMC11568179
- DOI: 10.1038/s41467-024-54293-z
Urease-powered nanomotor containing STING agonist for bladder cancer immunotherapy
Abstract
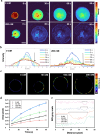
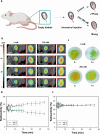
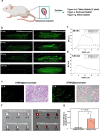
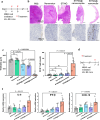
Most non-muscle invasive bladder cancers have been treated by transurethral resection and following intravesical injection of immunotherapeutic agents. However, the delivery efficiency of therapeutic agents into bladder wall is low due to frequent urination, which leads to the failure of treatment with side effects. Here, we report a urease-powered nanomotor containing the agonist of stimulator of interferon genes (STING) for the efficient activation of immune cells in the bladder wall. After characterization, we perform in vitro motion analysis and assess in vivo swarming behaviors of nanomotors. The intravesical instillation results in the effective penetration and retention of nanomotors in the bladder. In addition, we confirm the anti-tumor effect of nanomotor containing the STING agonist (94.2% of inhibition), with recruitment of CD8+ T cells (11.2-fold compared with PBS) and enhanced anti-tumor immune responses in bladder cancer model in female mice. Furthermore, we demonstrate the better anti-tumor effect of nanomotor containing the STING agonist than those of the gold standard Bacille Calmette-Guerin therapy and the anti-PD-1 inhibitor pembrolizumab in bladder cancer model. Taken together, the urease-powered nanomotor would provide a paradigm as a next-generation platform for bladder cancer immunotherapy.
© 2024. The Author(s).
Conflict of interest statement
Figures


References
-
- Cumberbatch, M. G. et al. Repeat transurethral resection in non-muscle-invasive bladder cancer: a systematic review. Eur. Urol.73, 925–933 (2018). - PubMed
-
- Kamat, M. et al. Bladder cancer. Lancet388, 2796–2810 (2016). - PubMed
-
- Tan, W. S., Rodney, S., Lamb, B., Feneley, M. & Kelly, J. Management of non-muscle invasive bladder cancer: a comprehensive analysis of guidelines from the United States, Europe and Asia. Cancer Treat. Rev.47, 22–31 (2016). - PubMed
-
- Pettenati, C. & Ingersoll, M. A. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat. Rev. Urol.15, 615–625 (2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

