Comparing stem cell mobilization with chemotherapy and cytokine (G-CSF) versus cytokine alone in myeloma patients (MOCCCA): a randomized phase II, open-label, non-inferiority trial
- PMID: 39548306
- PMCID: PMC11893443
- DOI: 10.1038/s41409-024-02468-z
Comparing stem cell mobilization with chemotherapy and cytokine (G-CSF) versus cytokine alone in myeloma patients (MOCCCA): a randomized phase II, open-label, non-inferiority trial
Abstract
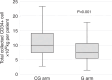
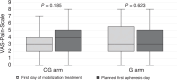
In fit patients with newly diagnosed myeloma, high-dose chemotherapy (HDCT) followed by autologous stem cell transplantation (ASCT) is considered standard of care. For mobilization of CD34+ cells for ASCT, combined cytotoxic chemotherapy and G-CSF is commonly used. However, the importance of cytostatic chemotherapy for reliable mobilization remains unclear. This prospective randomized phase II non-inferiority trial compared G-GSF only (G) compared to standard chemotherapy/G-CSF (CG) for CD34+ mobilization. The primary endpoint was a less than 15% difference in successful stem cell collection ( ≥ 5.0 × 106 CD34+ cells/kg b.w. in a single day collection procedure without additional stimulation with plerixafor) with the G regimen. 136 patients were 1:1 randomized. With an 18% difference in favor of the CG therapy, the non-inferiority margin was not maintained (95% CI 1%, 34%, p = 0.04). The median total CD34+ yield was 9.99 × 106/kg b.w. in CG patients and 7.42 × 106/kg b.w. in patients with G-CSF alone (p < 0.001). Ultimately, 130 (96%) patients proceeded to HDCT with ASCT. There were no differences in adverse events, hematologic engraftment, quality of life, or pain perception between the groups. Our data indicate that G-CSF only is inferior to chemotherapy with G-CSF for peripheral CD34+ stem cell mobilization. Trial registration SNCTP #: SNCTP000002952; Trials.gov #: NCT03442673.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: MD received financial support for attending haematology meetings from Kite Gilead/Novartis and serves as an advisory board member for Novartis. There are no financial disclosures from any of the other authors. Ethics approval and consent to participate: We confirm that all methods were performed in accordance with the relevant guidelines and regulations. The study had approval by the local Ethics Committee of Bern, Switzerland, with the decision number 2018-00615. Written informed consent was obtained from all participants.
Figures

Similar articles
-
Stem Cell Mobilization Yields with Daratumumab (Dara) and Lenalidomide (Len)-Containing Quadruplet Induction Therapy in Patients with Newly Diagnosed Multiple Myeloma (NDMM): A Real-World Experience at 2 Institutes.Clin Lymphoma Myeloma Leuk. 2025 Aug;25(8):e563-e569. doi: 10.1016/j.clml.2025.04.003. Epub 2025 Apr 11. Clin Lymphoma Myeloma Leuk. 2025. PMID: 40340128
-
Moving Beyond G-CSF Mobilization-Learning From a 15-Year Experience of Different Stem Cell Mobilization Regimens in Multiple Myeloma.Cancer Med. 2025 Jul;14(14):e71068. doi: 10.1002/cam4.71068. Cancer Med. 2025. PMID: 40667650 Free PMC article.
-
Efficacy and safety of etoposide + cytarabine + pegfilgrastim mobilization regimen versus G-CSF mobilization regimen alone for hematopoietic stem cell mobilization in patients with multiple myeloma and lymphoma.Cytotherapy. 2025 Aug;27(8):973-979. doi: 10.1016/j.jcyt.2025.05.012. Epub 2025 Jun 7. Cytotherapy. 2025. PMID: 40679461
-
Granulocyte colony-stimulating factor with or without stem or progenitor cell or growth factors infusion for people with compensated or decompensated advanced chronic liver disease.Cochrane Database Syst Rev. 2023 Jun 6;6(6):CD013532. doi: 10.1002/14651858.CD013532.pub2. Cochrane Database Syst Rev. 2023. PMID: 37278488 Free PMC article.
-
Efficacy of hematopoietic stem cell mobilization regimens in patients with hematological malignancies: a systematic review and network meta-analysis of randomized controlled trials.Stem Cell Res Ther. 2022 Mar 22;13(1):123. doi: 10.1186/s13287-022-02802-6. Stem Cell Res Ther. 2022. PMID: 35317856 Free PMC article.
References
-
- Dimopoulos MA, Moreau P, Terpos E, Mateos MV, Zweegman S, Cook G, et al. Multiple myeloma: EHA-ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2021;32:309–22. - PubMed

