Effect of nicotinamide riboside on airway inflammation in COPD: a randomized, placebo-controlled trial
- PMID: 39548320
- PMCID: PMC11645284
- DOI: 10.1038/s43587-024-00758-1
Effect of nicotinamide riboside on airway inflammation in COPD: a randomized, placebo-controlled trial
Abstract
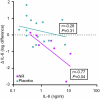
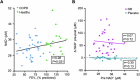
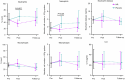
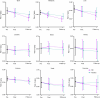
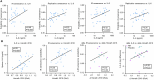
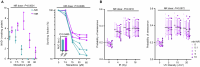
Chronic obstructive pulmonary disease (COPD) is a progressive, incurable disease associated with smoking and advanced age, ranking as the third leading cause of death worldwide. DNA damage and loss of the central metabolite nicotinamide adenine dinucleotide (NAD+) may contribute to both aging and COPD, presenting a potential avenue for interventions. In this randomized, double-blind, placebo-controlled clinical trial, we treated patients with stable COPD (n = 40) with the NAD+ precursor nicotinamide riboside (NR) for 6 weeks and followed-up 12 weeks later. The primary outcome was change in sputum interleukin-8 (IL-8) from baseline to week 6. The estimated treatment difference between NR and placebo in IL-8 after 6 weeks was -52.6% (95% confidence interval (CI): -75.7% to -7.6%; P = 0.030). This effect persisted until the follow-up 12 weeks after the end of treatment (-63.7%: 95% CI -85.7% to -7.8%; P = 0.034). For secondary outcomes, NR treatment increased NAD+ levels by more than twofold in whole blood, whereas IL-6 levels in plasma remained unchanged. In exploratory analyses, treatment with NR showed indications of upregulated gene pathways related to genomic integrity in the airways and reduced epigenetic aging, possibly through a reduction in cellular senescence. These exploratory analyses need to be confirmed in future trials. ClinicalTrials.gov identifier: NCT04990869 .
© 2024. The Author(s).
Conflict of interest statement
Competing interests: D.S. and R.W.D. are employees of Elysium Health and own shares in the company. The other authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

