Tetrandrine induces muscle atrophy involving ROS-mediated inhibition of Akt and FoxO3
- PMID: 39548359
- PMCID: PMC11566300
- DOI: 10.1186/s10020-024-00981-x
Tetrandrine induces muscle atrophy involving ROS-mediated inhibition of Akt and FoxO3
Abstract
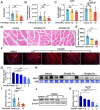
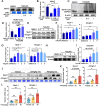
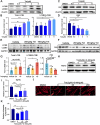
Tetrandrine (Tet), a well-known drug of calcium channel blocker, has been broadly applied for anti-inflammatory and anti-fibrogenetic therapy. However, due to the functional diversity of ubiquitous calcium channels, potential side-effects may be expected. Our previous report revealed an inhibitory effect of Tet on myogenesis of skeletal muscle. Here, we found that Tet induced protein degradation resulting in the myofibril atrophy. Upon administration with a relative high dose (40 mg/kg) of Tet for 28 days, the mice displayed significantly reduced muscle mass, strength force, and myosin heavy chain (MyHC) protein levels. The MyHC reduction was further detected in C2C12 myotubes after treating with Tet. Interestingly, the expression of Atrogin-1 and Murf-1, the skeletal muscle specific E3 ligases of protein ubiquitin-proteasome system (UPS), was accordingly up-regulated, and the reduced MyHC was significantly mitigated by MG132, a 26S proteasome inhibitor, indicating a key role of UPS in the protein degradation of muscle cells. Further study showed that Tet induced autophagy also participated in the protein degradation. Mechanistically, Tet treatment caused ROS production in myotubes that in turn targeted on FoxO3/AKT signaling, resulting in the activation of UPS and autophagy processes that were involved in the protein degradation. Our study reveals a potential side-effect of Tet on skeletal muscle atrophy, particularly when the drug dose is relatively high.
Keywords: Degradation; FoxO3/Akt; Myosin heavy chain; ROS; Tetrandrine.
© 2024. The Author(s).
Conflict of interest statement
Figures



References
-
- Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294(5547):1704–8. - PubMed
-
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–68. - PubMed
-
- Cai Y, Qi XM, Gong LK, Liu LL, Chen FP, Xiao Y, et al. Tetrandrine-induced apoptosis in rat primary hepatocytes is initiated from mitochondria: caspases and endonuclease G (Endo G) pathway. Toxicology. 2006;218(1):1–12. - PubMed
-
- Chandrashekar KR, Bhagya N, Ashwini Prabhu PD, Rekha. Tetrandrine isolated from Cyclea peltata induces cytotoxicity and apoptosis through ROS and caspase pathways in breast and pancreatic cancer cells. In Vitro Cell Dev Biol Anim. 2019;55(5):331–40. - PubMed
-
- Clarke BA, Drujan D, Willis MS, Murphy LO, Corpina RA, Burova E, et al. The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab. 2007;6(5):376–85. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

