An optimized Langendorff-free method for isolation and characterization of primary adult cardiomyocytes
- PMID: 39548395
- PMCID: PMC11566262
- DOI: 10.1186/s12872-024-04256-5
An optimized Langendorff-free method for isolation and characterization of primary adult cardiomyocytes
Abstract
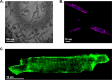
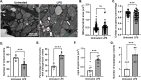
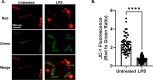
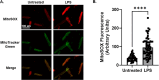
Isolation of adult mouse cardiomyocytes is an essential technique for advancing our understanding of cardiac physiology and pathology, and for developing therapeutic strategies to improve cardiac health. Traditionally, cardiomyocytes are isolated from adult mouse hearts using the Langendorff perfusion method in which the heart is excised, cannulated, and retrogradely perfused through the aorta. While this method is highly effective for isolating cardiomyocytes, it requires specialized equipment and technical expertise. To address the challenges of the Langendorff perfusion method, researchers have developed a Langendorff-free technique for isolating cardiomyocytes. This Langendorff-free technique involves anterograde perfusion through the coronary vasculature by clamping the aorta and intraventricular injection. This method simplifies the experimental setup by decreasing the need for specialized equipment and eliminating the need for cannulation of the heart. Here, we introduce an updated Langendorff-free method for isolating adult mice cardiomyocytes that builds on the Langendorff-free protocols developed previously. In this method, the aorta is clamped in situ, and the heart is perfused using a peristaltic pump, water bath, and an injection needle. This simplicity makes cardiomyocyte isolation more accessible for researchers who are new to cardiomyocyte isolation or are working with limited resources. In this report, we provide a step-by-step description of our optimized protocol. In addition, we present example studies of analyzing mitochondrial structural and functional characteristics in untreated isolated cardiomyocytes and cardiomyocytes treated with the acute inflammatory stimulus lipopolysaccharide (LPS).
Keywords: Cardiomyocytes; Heart function; Inflammation; Mitochondria; ROS; Sepsis.
© 2024. The Author(s).
Conflict of interest statement
Figures

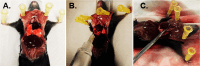
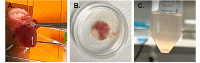

Update of
-
An Optimized Langendorff-free Method for Isolation and Characterization of Primary Adult Cardiomyocytes.Res Sq [Preprint]. 2024 Mar 29:rs.3.rs-4131724. doi: 10.21203/rs.3.rs-4131724/v1. Res Sq. 2024. Update in: BMC Cardiovasc Disord. 2024 Nov 15;24(1):649. doi: 10.1186/s12872-024-04256-5. PMID: 38585995 Free PMC article. Updated. Preprint.
Similar articles
-
An Optimized Langendorff-free Method for Isolation and Characterization of Primary Adult Cardiomyocytes.Res Sq [Preprint]. 2024 Mar 29:rs.3.rs-4131724. doi: 10.21203/rs.3.rs-4131724/v1. Res Sq. 2024. Update in: BMC Cardiovasc Disord. 2024 Nov 15;24(1):649. doi: 10.1186/s12872-024-04256-5. PMID: 38585995 Free PMC article. Updated. Preprint.
-
Isolation of Adult Mouse Cardiomyocytes Using Langendorff Perfusion Apparatus.Methods Mol Biol. 2021;2319:143-152. doi: 10.1007/978-1-0716-1480-8_16. Methods Mol Biol. 2021. PMID: 34331252 Free PMC article.
-
An Antegrade Perfusion Method for Cardiomyocyte Isolation from Mice.J Vis Exp. 2021 May 19;(171). doi: 10.3791/61866. J Vis Exp. 2021. PMID: 34096909
-
The isolated, perfused working heart preparation of the mouse-Advantages and pitfalls.Acta Physiol (Oxf). 2025 Apr;241(4):e70023. doi: 10.1111/apha.70023. Acta Physiol (Oxf). 2025. PMID: 40078031 Free PMC article. Review.
-
Retrograde heart perfusion: the Langendorff technique of isolated heart perfusion.J Mol Cell Cardiol. 2011 Jun;50(6):940-50. doi: 10.1016/j.yjmcc.2011.02.018. Epub 2011 Mar 6. J Mol Cell Cardiol. 2011. PMID: 21385587 Review.
Cited by
-
Myocardial pyruvate dehydrogenase kinase 4 drives sex-specific cardiac responses to endotoxemia.JCI Insight. 2025 Jul 8;10(13):e191649. doi: 10.1172/jci.insight.191649. eCollection 2025 Jul 8. JCI Insight. 2025. PMID: 40626362 Free PMC article.
-
Mitochondria at the Heart of Sepsis: Mechanisms, Metabolism, and Sex Differences.Int J Mol Sci. 2025 Apr 29;26(9):4211. doi: 10.3390/ijms26094211. Int J Mol Sci. 2025. PMID: 40362448 Free PMC article. Review.
-
Age-Related Mitochondrial Alterations Contribute to Myocardial Responses During Sepsis.Cells. 2025 Aug 7;14(15):1221. doi: 10.3390/cells14151221. Cells. 2025. PMID: 40801652 Free PMC article.
References
-
- Zimmer HG. The isolated perfused heart and its pioneers. News Physiol Sci. 1998;13:203–10. 10.1152/physiologyonline.1998.13.4.203. Epub 2001/06/08. - PubMed
-
- Omatsu-Kanbe M, Fukunaga R, Mi X, Matsuura H. An Antegrade Perfusion Method for Cardiomyocyte Isolation from Mice. J Vis Exp. 2021(171). Epub 2021/06/08. 10.3791/61866. PubMed PMID: 34096909. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

