CKS2 induces autophagy-mediated glutathione metabolic reprogramming to facilitate ferroptosis resistance in colon cancer
- PMID: 39548421
- PMCID: PMC11568617
- DOI: 10.1186/s10020-024-00979-5
CKS2 induces autophagy-mediated glutathione metabolic reprogramming to facilitate ferroptosis resistance in colon cancer
Erratum in
-
Correction: CKS2 induces autophagy-mediated glutathione metabolic reprogramming to facilitate ferroptosis resistance in colon cancer.Mol Med. 2025 Jan 8;31(1):6. doi: 10.1186/s10020-024-01041-0. Mol Med. 2025. PMID: 39780088 Free PMC article. No abstract available.
Abstract
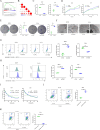
Background: Ferroptosis, a form of cell death characterized by lipid peroxidation, plays a crucial role in tumor suppression, offering novel avenues for cancer therapy. Previous studies have indicated that high levels of cyclin-dependent kinase subunit 2 (CKS2) promote the progression of various cancers. However, the potential interplay between CKS2 and ferroptosis in colon cancer (CC) remains unclear.
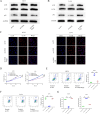
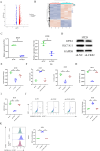
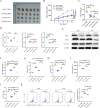
Methods: Bioinformatics and RNA-seq analyses were employed to study genes associated with the ferroptosis signaling pathway. CKS2 expression was evaluated using quantitative reverse transcription polymerase chain reaction (qRT-PCR) and Western blot (WB). The in vitro and in vivo effects of CKS2 on CC cells were assessed through the CCK-8 assay, colony formation assay, propidium iodide (PI) staining, BODIPY staining, DCFH-DA staining, and animal experiments. Additionally, the impact of CKS2 on autophagy and glutathione (GSH) metabolism was investigated using a transmission electron microscope (TEM), immunofluorescence (IF) assays, WB experiments, and relevant assay kits.
Results: CKS2 expression was elevated in CC, indicating a poor clinical outcome. Knockdown of CKS2 significantly enhanced Erastin-induced ferroptosis in CC cells, leading to reduced GSH metabolism. Conversely, CKS2 overexpression produced opposite effects. Mechanistically, CKS2-induced autophagy reinforced GSH metabolism, thereby increasing resistance to ferroptosis in CC cells. Furthermore, inhibiting CKS2 promoted tumor ferroptosis by downregulating GPX4 expression. Additionally, CKS2 knockdown effectively increased sorafenib-induced ferroptosis both in vitro and in vivo.
Conclusion: CKS2 suppresses ferroptosis in CC by modulating GSH metabolism in both in vitro and in vivo settings. These findings offer new insights into targeting CKS2 for CC treatment and shed light on the mechanism of ferroptosis in CC.
Keywords: Autophagy; Colon cancer; Cyclin-dependent kinase subunit 2; Ferroptosis; Glutathione metabolism.
© 2024. The Author(s).
Conflict of interest statement
Figures




Similar articles
-
CKS2 Promotes the Growth in Non-Small-Cell Lung Cancer by Downregulating Cyclin-Dependent Kinase Inhibitor.Pathobiology. 2022;89(1):13-22. doi: 10.1159/000517755. Epub 2021 Jul 30. Pathobiology. 2022. PMID: 34333494
-
Cyclin-dependent kinase subunit2 (CKS2) promotes malignant phenotypes and epithelial-mesenchymal transition-like process in glioma by activating TGFβ/SMAD signaling.Cancer Med. 2023 Mar;12(5):5889-5907. doi: 10.1002/cam4.5381. Epub 2022 Oct 25. Cancer Med. 2023. PMID: 36284444 Free PMC article.
-
Oncogenic potential of cyclin kinase subunit-2 in cholangiocarcinoma.Liver Int. 2013 Jan;33(1):137-48. doi: 10.1111/liv.12014. Epub 2012 Nov 1. Liver Int. 2013. PMID: 23121546
-
Aberrant expression of CKS2 induced by ELK1 contributes to malignant progression of pancreatic cancer.Mol Carcinog. 2023 Dec;62(12):1947-1959. doi: 10.1002/mc.23627. Epub 2023 Aug 29. Mol Carcinog. 2023. PMID: 37642304
-
Tumor Metabolic Reprogramming and Ferroptosis: The Impact of Glucose, Protein, and Lipid Metabolism.Int J Mol Sci. 2024 Dec 14;25(24):13413. doi: 10.3390/ijms252413413. Int J Mol Sci. 2024. PMID: 39769177 Free PMC article. Review.
Cited by
-
Potential mechanism of Camellia luteoflora against colon adenocarcinoma: An integration of network pharmacology and molecular docking.World J Gastrointest Oncol. 2025 Jun 15;17(6):105782. doi: 10.4251/wjgo.v17.i6.105782. World J Gastrointest Oncol. 2025. PMID: 40547172 Free PMC article.
-
Correction: CKS2 induces autophagy-mediated glutathione metabolic reprogramming to facilitate ferroptosis resistance in colon cancer.Mol Med. 2025 Jan 8;31(1):6. doi: 10.1186/s10020-024-01041-0. Mol Med. 2025. PMID: 39780088 Free PMC article. No abstract available.
References
-
- Chen Y, Zhu G, Liu Y, Wu Q, Zhang X, Bian Z, et al. O-GlcNAcylated c-Jun antagonizes ferroptosis via inhibiting GSH synthesis in liver cancer. Cell Signal. 2019;63:109384. - PubMed
-
- Chen X, Kang R, Kroemer G, Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat Reviews Clin Oncol. 2021;18:280–96. - PubMed
-
- Chen L, Hu K, Liu Y, Liu L, Tang J, Qin X. Knockdown of replication protein A 3 induces protective autophagy and enhances cisplatin sensitivity in lung adenocarcinoma by inhibiting AKT/mTOR signaling via binding to cyclin-dependent kinases regulatory subunit 2. Drug Dev Res. 2022a;83:1589–99. - PubMed
-
- Chen Y, Lin B, Yang S, Huang J. IRF1 suppresses colon cancer proliferation by reducing SPI1-mediated transcriptional activation of GPX4 and promoting ferroptosis. Exp Cell Res. 2023;431:113733. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

