Gut microbial and metabolomics profiles reveal the potential mechanism of fecal microbiota transplantation in modulating the progression of colitis-associated colorectal cancer in mice
- PMID: 39548468
- PMCID: PMC11566892
- DOI: 10.1186/s12967-024-05786-4
Gut microbial and metabolomics profiles reveal the potential mechanism of fecal microbiota transplantation in modulating the progression of colitis-associated colorectal cancer in mice
Abstract
Purpose: Intestinal flora promotes the pathogenesis of colorectal cancer (CRC) through microorganisms and their metabolites. This study aimed to investigate the composition of intestinal flora in different stages of CRC progression and the effect of fecal microbiota transplantation (FMT) on CRC mice.
Methods: The fecal microbiome from healthy volunteers (HC), colorectal adenoma (CRA), inflammatory bowel disease (IBD), and CRC patients were analyzed by 16s rRNA gene sequencing. In an azoxymethane (AOM)/dextran-sulfate-sodium (DSS)-induced CRC mouse, the effect of FMT from HC, CRA, CRC, and IBD patients on CRC mice was assessed by histological analysis. Expression of inflammation- EMT-associated proteins and Wnt/β-catenin pathway were assessed using qRT-PCR and western blot. The ratio of the fecal microorganisms and metabolomics alteration after FMT were also assessed.
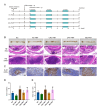
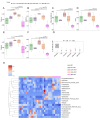
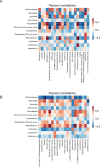
Result: Prevotella, Faecalibacterium, Phascolarctobacterium, Veillonella, Alistipes, Fusobacterium, Oscillibacter, Blautia, and Ruminococcus abundance was different among HC, IBD, CRC, and CRA patients. HC-FMT alleviated disease progression and inflammatory response in CRC mice, inhibited splenic T help (Th)1 and Th17 cell numbers, and suppressed the EMT and Wnt/β-catenin pathways in tumor tissues of CRC mice. IBD-FMT, CRA-FMT, and CRC-FMT played deleterious roles; the CRC-FMT mice exhibited the most malignant phenotype. Compared with the non-FMT CRC mice, Muribaculaceae abundance was lower after FMT, especially lowest in the IBD-FMT group; while Lactobacillus abundance was higher after FMT and especially high in HC-FMT. Akkermansia and Ileibacterium abundance increased after FMT-HC compared to other groups. Metabolite correlation analysis revealed that Muribaculaceae abundance was significantly correlated with metabolites such as Betaine, LysoPC, and Soyasaponin III. Lactobacillus abundance was positively correlated with Taurocholic acid 3-sulfate, and Ileibacterium abundance was positively correlated with Linoleoyl ethanolamide.
Conclusion: The different intestinal microbiota communities of HC, IBD, CRA, and CRC patients may be attributed to the different modulation effects of FMT on CRC mice. CRC-FMT promoted, while HC-FMT inhibited the progress of CRC. Increased linoleoyl ethanolamide levels and abundance of Muribaculaceae, Akkermansia, and Ileibacterium and reduced Fusobacterium might participate in inhibiting CRC initiation and development. This study demonstrated that FMT intervention could restore the intestinal microbiota and metabolomics of CRC mice, suggesting FMT as a potential strategy for CRC therapy.
Keywords: 16s rRNA gene sequencing; Enteritis-associated colorectal cancer; Epithelial-mesenchymal transition (EMT); Fecal microbiota transplantation; Metabolomics; Th1/Th17 cells; Wnt/β-catenin pathway.
© 2024. The Author(s).
Conflict of interest statement
Figures






Similar articles
-
Curcumin suppresses colorectal tumorigenesis through restoring the gut microbiota and metabolites.BMC Cancer. 2024 Sep 12;24(1):1141. doi: 10.1186/s12885-024-12898-z. BMC Cancer. 2024. PMID: 39267014 Free PMC article.
-
Integration of microbiome, metabolomics and transcriptome for in-depth understanding of berberine attenuates AOM/DSS-induced colitis-associated colorectal cancer.Biomed Pharmacother. 2024 Oct;179:117292. doi: 10.1016/j.biopha.2024.117292. Epub 2024 Aug 15. Biomed Pharmacother. 2024. PMID: 39151314
-
Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii served as key components of fecal microbiota transplantation to alleviate colitis.Am J Physiol Gastrointest Liver Physiol. 2024 May 1;326(5):G607-G621. doi: 10.1152/ajpgi.00303.2023. Epub 2024 Mar 19. Am J Physiol Gastrointest Liver Physiol. 2024. PMID: 38502145 Free PMC article.
-
Gut microbiota alterations in colorectal adenoma-carcinoma sequence based on 16S rRNA gene sequencing: A systematic review and meta-analysis.Microb Pathog. 2024 Oct;195:106889. doi: 10.1016/j.micpath.2024.106889. Epub 2024 Aug 26. Microb Pathog. 2024. PMID: 39197689
-
Gut microbiome, metabolome, host immunity associated with inflammatory bowel disease and intervention of fecal microbiota transplantation.J Autoimmun. 2023 Dec;141:103062. doi: 10.1016/j.jaut.2023.103062. Epub 2023 May 27. J Autoimmun. 2023. PMID: 37246133 Review.
Cited by
-
Advances in research on the intestinal microbiota in the mechanism and prevention of colorectal cancer (Review).Mol Med Rep. 2025 May;31(5):133. doi: 10.3892/mmr.2025.13498. Epub 2025 Mar 21. Mol Med Rep. 2025. PMID: 40116116 Free PMC article. Review.
-
Exploring the etiology of colitis: insights from gut microbiota research.Gut Microbes. 2025 Dec;17(1):2512010. doi: 10.1080/19490976.2025.2512010. Epub 2025 Jun 2. Gut Microbes. 2025. PMID: 40457634 Free PMC article. Review.
-
Metabolic interactions: how gut microbial metabolites influence colorectal cancer.Front Microbiol. 2025 Jul 23;16:1611698. doi: 10.3389/fmicb.2025.1611698. eCollection 2025. Front Microbiol. 2025. PMID: 40771692 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

