Efficacy and safety of novel multiple-chain DAP-CAR-T cells targeting mesothelin in ovarian cancer and mesothelioma: a single-arm, open-label and first-in-human study
- PMID: 39548510
- PMCID: PMC11568615
- DOI: 10.1186/s13073-024-01405-5
Efficacy and safety of novel multiple-chain DAP-CAR-T cells targeting mesothelin in ovarian cancer and mesothelioma: a single-arm, open-label and first-in-human study
Abstract
Background: Despite remarkable achievements in applying chimeric antigen receptor (CAR)-T cells to treat hematological malignancies, they remain much less effective against solid tumors, facing several challenges affecting their clinical use. We previously showed that multichain DNAX-activating protein (DAP) CAR structures could enhance the safety and efficacy of CAR-T cells when used against solid tumors. In particular, mesothelin (MSLN)-targeted CAR-T cell therapy has therapeutic potential in MSLN-positive solid tumors, including ovarian cancer and mesothelioma.
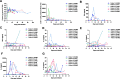
Methods: In vitro cell killing assays and xenograft model were utilized to determine the anti-tumor efficacy of MSLN targeting DAP-CAR-T cells and other CAR-T cells. ELISA and flow cytometry analysis were used to assess the cytokine secretion capacity and proliferation ability. Eight patients with MSLN expression were enrolled to evaluate the safety and efficacy of MSLN-DAP CAR-T cell therapy. Single-cell sequencing was performed to explore the dynamics of immune cells in patients during treatment and to identify the transcriptomic signatures associated with efficacy and toxicity.
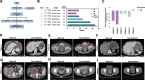
Results: We found that multichain DAP-CAR formed by combining a natural killer cell immunoglobulin-like receptor truncator and DAP12 exhibited better cytotoxicity and tumor-killing capacity than other natural killer cell-activated receptors associated with DAP12, DAP10, or CD3Z. The safety and efficacy of MSLN-DAP CAR-T cell therapy in patients with ovarian cancer and mesothelioma were evaluated in a single-arm, open-label clinical trial (ChiCTR2100046544); two patients achieved partial response, while four patients had a stable disease status. Furthermore, single-cell sequencing analysis indicated that KT032 CAR-T cell infusion could recruit more immune cells and temporarily remodel the TME.
Conclusions: Our study highlights the safety and therapeutic efficacy of multiple-chain DAP-CAR-T cell therapy targeting MSLN to treat patients with ovarian cancer and mesothelioma.
Trial registration: ChiCTR.org.cn, ChiCTR2100046544 . May 21, 2021.
Keywords: CAR-T cells; Efficacy; MSLN; Mesothelioma; Ovarian cancer; Safety.
© 2024. The Author(s).
Conflict of interest statement
Figures




Similar articles
-
Potent and durable control of mesothelin-expressing tumors by a novel T cell-secreted bi-specific engager.J Immunother Cancer. 2025 Mar 13;13(3):e010063. doi: 10.1136/jitc-2024-010063. J Immunother Cancer. 2025. PMID: 40081946 Free PMC article.
-
Disruption of adenosine 2A receptor improves the anti-tumor function of anti-mesothelin CAR T cells both in vitro and in vivo.Exp Cell Res. 2021 Dec 1;409(1):112886. doi: 10.1016/j.yexcr.2021.112886. Epub 2021 Oct 19. Exp Cell Res. 2021. PMID: 34673000
-
Use of chimeric antigen receptor NK-92 cells to target mesothelin in ovarian cancer.Biochem Biophys Res Commun. 2020 Mar 26;524(1):96-102. doi: 10.1016/j.bbrc.2020.01.053. Epub 2020 Jan 22. Biochem Biophys Res Commun. 2020. PMID: 31980173
-
Mesothelin-targeted CAR-T cell therapy for solid tumors.Expert Opin Biol Ther. 2021 Apr;21(4):473-486. doi: 10.1080/14712598.2021.1843628. Epub 2020 Nov 12. Expert Opin Biol Ther. 2021. PMID: 33176519 Review.
-
Mesothelin as a target for chimeric antigen receptor-modified T cells as anticancer therapy.Immunotherapy. 2016;8(4):449-60. doi: 10.2217/imt.16.4. Immunotherapy. 2016. PMID: 26973126 Free PMC article. Review.
Cited by
-
Therapeutic landscape of ovarian cancer: recent advances and emerging therapies.Biomark Res. 2025 Aug 12;13(1):103. doi: 10.1186/s40364-025-00818-7. Biomark Res. 2025. PMID: 40797342 Free PMC article. Review.
References
-
- Holstein SA, Lunning MA. CAR T-cell therapy in hematologic malignancies: a voyage in progress. Clin Pharmacol Ther. 2020;107:112–22. - PubMed
-
- San-Miguel J, Dhakal B, Yong K, Spencer A, Anguille S, Mateos MV, Fernandez de Larrea C, Martinez-Lopez J, Moreau P, Touzeau C, et al. Cilta-cel or standard care in lenalidomide-refractory multiple myeloma. N Engl J Med. 2023;389:335–47. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

