CXCL11 reprograms M2-biased macrophage polarization to alleviate pulmonary fibrosis in mice
- PMID: 39548525
- PMCID: PMC11566568
- DOI: 10.1186/s13578-024-01320-7
CXCL11 reprograms M2-biased macrophage polarization to alleviate pulmonary fibrosis in mice
Abstract
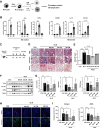
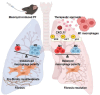
Background: In understanding the pathophysiology of pulmonary fibrosis (PF), macrophage plasticity has been implicated with a crucial role in the fibrogenic process. Growing evidence indicates that accumulation of M2 macrophages correlates with the progression of PF, suggesting that targeted modulation of molecules that influence M2 macrophage polarization could be a promising therapeutic approach for PF. Here, we demonstrated a decisive role of C-X-C motif chemokine ligand 11 (CXCL11) in driving M1 macrophage polarization to alleviate PF in the bleomycin-induced murine model.
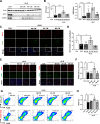
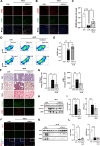
Results: We intravenously administered secretome derived from naïve (M0) and polarized macrophages (M1 and M2) into PF mice and found that lung fibrosis was effectively reversed in only the M1-treated group, with modulation of the M1/M2 ratio toward the ratio of the control group. These findings suggest that the factors secreted from M1 macrophages contribute to alleviating PF by targeting macrophages and reshaping the immunofibrotic environment in a paracrine manner. Secretome analysis of macrophages identified CXCL11 as an M1-specific chemokine, and administration of recombinant CXCL11 effectively improved fibrosis with the reduction of M2 macrophages in vivo. Furthermore, a mechanistic in vitro study revealed that CXCL11 reprogrammed macrophages from M2 to M1 through the activation of pERK, pAKT, and p65 signaling.
Conclusions: Collectively, we demonstrate an unprecedented role for M1 macrophage-derived CXCL11 as an inducer of M1 macrophage polarization to revert the fibrogenic process in mice with PF, which may provide a clinically meaningful benefit.
Keywords: CXCL11; Macrophage; Polarization; Pulmonary fibrosis.
© 2024. The Author(s).
Conflict of interest statement
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

