Cytomegalovirus infection of the fetal brain: intake of aspirin during pregnancy blunts neurodevelopmental pathogenesis in the offspring
- PMID: 39548550
- PMCID: PMC11566200
- DOI: 10.1186/s12974-024-03276-4
Cytomegalovirus infection of the fetal brain: intake of aspirin during pregnancy blunts neurodevelopmental pathogenesis in the offspring
Abstract
Background: Congenital cytomegalovirus (CMV) infections represent one leading cause of human neurodevelopmental disorders. Despite their high prevalence and severity, no satisfactory therapy is available and pathophysiology remains elusive. The pathogenic involvement of immune processes occurring in infected developing brains has been increasingly documented. Here, we have used our previously validated rat model of CMV infection of the fetal brain in utero to test whether the maternal administration of four different drugs with immunomodulatory properties would have an impact on the detrimental postnatal outcome of CMV infection.
Methods: CMV infection of the rat fetal brain was done intracerebroventricularly. Each of the drugs, including acetylsalicylic acid (aspirin, ASA), a classical inhibitor of cyclooxygenases Cox-1 and Cox-2, the two key rate-limiting enzymes of the arachidonic acid-to-prostaglandins (PG) synthesis pathway, was administered to pregnant dams until delivery. ASA was selected for subsequent analyses based on the improvement in postnatal survival. A combination of qRT-PCR, mass spectrometry-based targeted lipidomics, immunohistochemistry experiments, monitoring of neurologic phenotypes and electrophysiological recordings was used to assess the impact of ASA in CMV-infected samples and pups. The postnatal consequences of CMV infection were also analyzed in rats knocked-out (KO) for Cox-1.
Results: Increased PGE2 levels and increased proportions of Cox-1+ and Cox-2+ microglia were detected in CMV-infected developing brains. Maternal intake of ASA led to decreased proportion of Cox-1+ fetal, but not neonatal, microglia, while leaving the proportions of Cox-2+ microglia unchanged. Maternal intake of ASA also improved the key postnatal in vivo phenotypes caused by CMV infection and dramatically prevented against the spontaneous epileptiform activity recorded in neocortical slices from CMV-infected pups. In contrast with maternal intake of ASA, Cox-1 KO pups displayed no improvement in the in vivo phenotypes after CMV infection. However, as with ASA administration, the spontaneous epileptiform activity was dramatically inhibited in neocortical slices from CMV-infected, Cox-1 KO pups.
Conclusion: Overall, our data indicate that, in the context of CMV infection of the fetal brain, maternal intake of ASA during pregnancy improved CMV-related neurodevelopmental alterations in the offspring, likely via both Cox-1 dependent and Cox-1 independent mechanisms, and provide proof-of-principle for the use of ASA against the detrimental outcomes of congenital CMV infections.
Keywords: CMV; Cox-1; Cyclooxygenase; Fetal brain; Herpes virus; Lipids.
© 2024. The Author(s).
Conflict of interest statement
Figures



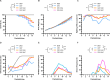
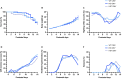


References
-
- Adler SP, Nigro G. Prevention of maternal-fetal transmission of cytomegalovirus. Clin Infect Dis. 2013;57(suppl_4):S189–92. 10.1093/cid/cit585. - PubMed
-
- Leruez-Ville M, Foulon I, Pass R, Ville Y. Cytomegalovirus infection during pregnancy: state of the science. Am J Obstet Gynecol. 2020;223(3):330–49. 10.1016/j.ajog.2020.02.018. - PubMed
-
- Børglum AD, Demontis D, Grove J, Pallesen J, Hollegaard MV, Pedersen CB, Hedemand A, Mattheisen M, Uitterlinden A, Nyegaard M, Ørntoft T, Wiuf C, Didriksen M, Nordentoft M, Nöthen MM, Rietschel M, Ophoff RA, Cichon S, Yolken RH, Mortensen PB, Mors O. Genome-wide study of association and interaction with maternal cytomegalovirus infection suggests new schizophrenia loci. Mol Psychiatry. 2014;19(3):325–33. 10.1038/mp.2013.2. - PMC - PubMed
-
- Piccirilli G, Gabrielli L, Bonasoni MP, Chiereghin A, Turello G, Borgatti EC, Simonazzi G, Felici S, Leone M, Salfi NCM, Santini D, Lazzarotto T. Fetal brain damage in human fetuses with congenital cytomegalovirus infection: histological features and viral tropism. Cell Mol Neurobiol. 2023;43(3):1385–99. 10.1007/s10571-022-01258-9. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- CALIN - R24002AA/Excellence Initiative of Aix-Marseille University/A*MIDEX grant
- CALIN - R24002AA/Excellence Initiative of Aix-Marseille University/A*MIDEX grant
- CALIN - R24002AA/Excellence Initiative of Aix-Marseille University/A*MIDEX grant
- CALIN - R24002AA/Excellence Initiative of Aix-Marseille University/A*MIDEX grant
- ANR-11-LABX-0021 (LipSTIC Labex)/French 'Investissements d'Avenir' programme
LinkOut - more resources
Full Text Sources
Medical
Research Materials

