An in vivo and in silico probing of the protective potential of betaine against sodium fluoride-induced neurotoxicity
- PMID: 39548593
- PMCID: PMC11568634
- DOI: 10.1186/s40360-024-00812-z
An in vivo and in silico probing of the protective potential of betaine against sodium fluoride-induced neurotoxicity
Abstract
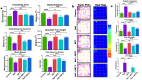
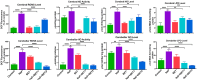
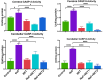
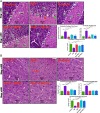
Excessive fluoride exposure beyond the tolerable limit may adversely impacts brain functionality. Betaine (BET), a trimethyl glycine, possesses antioxidant, anti-inflammatory and anti-apoptotic functions, although the underlying mechanisms of the role of BET on fluoride-induced neurotoxicity remain unelucidated. To assess the mechanism involved in the neuro-restorative role of BET on behavioural, neurochemical, and histological changes, we employed a rat model of sodium fluoride (NaF) exposure. Animals were treated with NaF (9 mg/kg) body weight (bw) only or co-treated with BET (50 and 100 mg/kg bw) orally uninterrupted for 28 days. We obtained behavioural phenotypes in an open field, performed negative geotaxis, and a forelimb grip test, followed by oxido-inflammatory, apoptotic, and histological assessment. Behavioural endpoints indicated lessened locomotive and motor and heightened anxiety-like performance and upregulated oxidative, inflammatory, and apoptotic biomarkers in NaF-exposed rats. Co-treatment with BET significantly enhanced locomotive, motor, and anxiolytic performance, increased the antioxidant signalling mechanisms and demurred oxidative, inflammatory, and apoptotic biomarkers and histoarchitectural damage in the cerebrum and cerebellum cortices mediated by NaF. The in-silico analysis suggests that multiple hydrogen bonds and hydrophobic interactions of BET with critical amino acid residues, including arginine (ARG380 and ARG415) in the Keap1 Kelch domain, which may disrupt Keap1-Nrf2 complex and activate Nrf2. This may account for the observed increased in the Nrf2 levels, elevated antioxidant response and enhanced anti-inflammatory response. The BET-Keap1 complex was also observed to exhibit structural stability and conformational flexibility in solvated biomolecular systems, as indicated by the thermodynamic parameters computed from the trajectories obtained from a 100 ns full atomistic molecular dynamics simulation. Therefore, BET mediates neuroprotection against NaF-induced cerebro-cerebellar damage through rats' antioxidant, anti-inflammatory, and anti-apoptotic activity, which molecular interactions with Keap1-Nrf2 may drive.
Keywords: Betaine; Molecular docking; Molecular dynamics simulation; Neurotoxicity; Oxidative stress; Sodium fluoride.
© 2024. The Author(s).
Conflict of interest statement
Figures










References
-
- Pretty IA. High fluoride concentration toothpastes for children and adolescents. Caries Res. 2016;50(Suppl 1):9–14. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

