Persistence of activated anti-mesothelin hYP218 chimeric antigen receptor T cells in the tumour is associated with efficacy in gastric and colorectal carcinomas
- PMID: 39548594
- PMCID: PMC11567854
- DOI: 10.1002/ctm2.70057
Persistence of activated anti-mesothelin hYP218 chimeric antigen receptor T cells in the tumour is associated with efficacy in gastric and colorectal carcinomas
Abstract
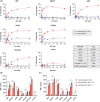
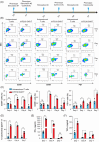
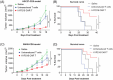
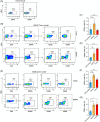
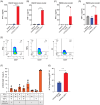
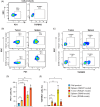
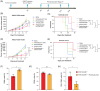
Patients with advanced gastric and colorectal cancers have limited treatment options. Since mesothelin is highly expressed in these tumour types, we evaluated the therapeutic benefits of anti-mesothelin hYP218 CAR T cells alone, and in combination with anti-PD1 antibody, pembrolizumab. GEPIA analysis was performed using human gastric (n = 408) and colon cancer tumours (n = 275) in TCGA database, to evaluate mRNA expression of mesothelin, compared to normal tissues. Mesothelin expression in gastric and colorectal cancer cell-lines (n = 5) was analysed using flow cytometry. In vitro efficacy by hYP218 CAR T cells was tested by cytotoxicity and cytokine release assays. In vivo anti-tumour efficacy of hYP218 CAR T cells alone, and in combination with pembrolizumab, was evaluated in NSG mice bearing human gastric (HGC27) and colorectal (SW48) tumour xenografts. Additionally, hYP218 CAR-T cell persistence, activation and exhaustion marker-expression were studied. Mesothelin expression was significantly higher in gastric and colon cancer biopsies compared to normal tissues (p < .005). Mesothelin expression in gastric and colon cancer cell lines ranged from 10 000 to 70 000 molecules per cell. hYP218 CAR T cells demonstrated strong cytotoxic activity at low effector to target ratio, ranging from 0.24 to 1.0. In NSG mouse-models, hYP218 CAR T cells demonstrated anti-tumour efficacy and persisted in the tumour microenvironment in a functional state at day 40 posttreatment with expression of activation markers CD39 and CD69, increased production of IFN-γ and TNF-α and ability to kill tumour cells in vitro when isolated from tumours. There was increased PD1 expression. In combination with pembrolizumab, hYP218 CAR T cells led to slower tumour growth in NSG mice bearing large but not small HGC27 tumours. Anti-tumour efficacy of hYP218 CAR T cells is due to increased accumulation of activated CAR T cells in the tumour and combination with pembrolizumab resulted in improvement in anti-tumour activity of large established tumours. HIGHLIGHTS: Mesothelin expression is significantly higher in gastric and colorectal cancers than normal tissues. hYP218 CAR T cells demonstrate strong anti-tumour activity against mesothelin-positive gastric and colorectal carcinomas. Activated hYP218 CAR T cells persist in the tumour microenvironment and retain their cytotoxic activity. Addition of pembrolizumab in larger tumours enhance CAR T cell efficacy.
Keywords: CAR T cell; anti‐PD1; colorectal cancer; gastric cancer; mesothelin; pembrolizumab; xenograft cancer model.
Published 2024. This article is a U.S. Government work and is in the public domain in the USA. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
RH has received funding from the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (ZIA‐BC‐010816) and has received funding for conduct of clinical trials via a cooperative research and development agreement between NCI and Bayer AG and TCR2 Therapeutics. Other authors have no competing interests to disclose.
Figures

References
-
- Hossain MS, Karuniawati H, Jairoun AA, et al. Colorectal cancer: a review of carcinogenesis, global epidemiology, current challenges, risk factors, preventive and treatment strategies. Cancers (Basel). 2022;14(7). doi:10.3390/cancers14071732. [published Online First: 2022/04/13]. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
