Using an experience-based co-design approach to develop strategies for implementing an intravenous iron intervention to treat moderate and severe anemia in pregnancy in Malawi
- PMID: 39548595
- PMCID: PMC11568636
- DOI: 10.1186/s43058-024-00661-1
Using an experience-based co-design approach to develop strategies for implementing an intravenous iron intervention to treat moderate and severe anemia in pregnancy in Malawi
Abstract
Background: In low- and middle-income countries, women experiencing anemia during pregnancy are recommended to take 30 mg to 60 mg of oral iron daily throughout pregnancy. However, oral iron tablets are often poorly tolerated and slow in correcting anemia, resulting in low adherence, prolonged anemia, and increased risk of adverse maternal and fetal outcomes. An alternative to oral iron is intravenous (IV) iron, commonly used in high-income countries to restore the body's iron stores rapidly. A randomized controlled trial was conducted to investigate the effectiveness and safety of IV iron compared to standard-of-care oral iron supplementation for pregnant women with moderate and severe anemia in the third trimester in Malawi (REVAMP-TT). Using an experience-based co-design approach, our study aimed to identify barriers and facilitators to IV iron use to treat anemia in pregnancy in the primary healthcare system of Malawi, and develop mitigating strategies for the successful implementation of REVAMP-TT.
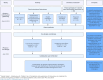
Methodology: The co-design process involved two phases: i) We conducted an information-gathering exercise to identify barriers and facilitators to IV iron use to treat anemia in pregnancy in the primary healthcare system of Malawi. We interviewed key informants (n = 53) including the policymakers, government partners, healthcare managers, and healthcare providers. We also gathered previous research findings from a formative qualitative study on the perceptions and experiences of IV iron treatment for pregnant women experiencing anemia in Malawi (n = 29). ii) We conducted two co-design workshops with end-users (n = 20) and healthcare providers (n = 20) to confirm and identify the key barriers and facilitators and developed mitigating strategies to inform the successful implementation of the REVAMP-TT trial. We mapped the emerging barriers to the Consolidated Framework for Implementation Research 2.0 (CFIR 2.0) and matched the mitigating strategies to the corresponding Expert Recommendations for Implementing Change (ERIC) compilation.
Results: The following were identified as key barriers to IV iron use to treat anemia in pregnancy in the primary healthcare system of Malawi: the cost of IV iron, the lack of available resources and knowledge, local attitudes including myths and misconceptions about IV iron and keeping pregnancy a secret, local conditions, the lack of political will and buy-in from high-level leaders, the lack of capability of healthcare providers to deliver IV iron, and the lack of male involvement to support pregnant women's access to antenatal care. The proposed strategies to mitigate the barriers for the successful implementation of the REVAMP TT trial included providing financial strategy, developing stakeholder relationships, training and educating stakeholders, supporting clinicians, and engaging end-users.
Conclusion: The use of the experience-based co-design approach in our study provided a valuable method to expose the potential barriers and facilitators to IV iron use and develop mitigating strategies to successfully implement the REVAMP-TT trial. Engaging both the key informants and end users promoted ownership and consensus among stakeholders and ensured a collaborative environment for sharing deeply rooted real-world experiences and insights. Not only do these findings address the needs of this study, but they also, lay a groundwork for the possible integration of IV iron into routine care in Malawi and provide knowledge for policymakers to make informed decisions on the management of anemia in the primary healthcare systems of Malawi.
Keywords: Anemia; Antenatal care; Community engagement; Experience-based co-design; Implementation strategies; Intravenous iron; Pregnant women.
© 2024. The Author(s).
Conflict of interest statement
Figures





Similar articles
-
An implementation research programme to support an intravenous iron intervention for pregnant women with moderate and severe anaemia in Malawi: study protocol.Implement Sci Commun. 2022 Jun 21;3(1):68. doi: 10.1186/s43058-022-00299-x. Implement Sci Commun. 2022. PMID: 35729604 Free PMC article.
-
Protocol and statistical analysis plan for a randomized controlled trial of the effect of intravenous iron on anemia in Malawian pregnant women in their third trimester (REVAMP - TT).Gates Open Res. 2023 Dec 18;7:117. doi: 10.12688/gatesopenres.14710.2. eCollection 2023. Gates Open Res. 2023. PMID: 38343768 Free PMC article.
-
Acceptability of IV iron treatment for iron deficiency anaemia in pregnancy in Nigeria: a qualitative study with pregnant women, domestic decision-makers, and health care providers.Reprod Health. 2024 Feb 13;21(1):22. doi: 10.1186/s12978-024-01743-y. Reprod Health. 2024. PMID: 38347614 Free PMC article.
-
Conceptual framework on barriers and facilitators to implementing perinatal mental health care and treatment for women: the MATRIx evidence synthesis.Health Soc Care Deliv Res. 2024 Jan;12(2):1-187. doi: 10.3310/KQFE0107. Health Soc Care Deliv Res. 2024. PMID: 38317290
-
Barriers and Facilitators to Implementing Interventions for Reducing Avoidable Hospital Readmission: Systematic Review of Qualitative Studies.Int J Health Policy Manag. 2023;12:7089. doi: 10.34172/ijhpm.2023.7089. Epub 2023 Feb 14. Int J Health Policy Manag. 2023. PMID: 37579466 Free PMC article.
References
-
- Vitamin and Mineral Nutrition Information System (VMNIS). WHO global database on Anaemia The database on Anaemia includes data by country on prevalence of anaemia and mean haemoglobin concentration. 2007.
-
- Vanobberghen F, Lweno O, Kuemmerle A, Mwebi KD, Asilia P, Issa A, et al. Efficacy and safety of intravenous ferric carboxymaltose compared with oral iron for the treatment of iron deficiency anaemia in women after childbirth in Tanzania: a parallel-group, open-label, randomised controlled phase 3 trial. Lancet Glob Health. 2021;9(2):e189–98. - PubMed
-
- Jericó C, Beverina I, Quintana-Diaz M, Salvadori U, Melli C, Rondinelli MB, Recasens V, Brando B, Garcia-Erce JA. Efficacy and safety of high-dose intravenous iron as the first-choice therapy in outpatients with severe iron deficiency anemia. Transfusion. 2020;60(7):1443–9. 10.1111/trf.15870. Epub 2020 Jun 29. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

