Blood metabolomic profile in patients with type 2 diabetes mellitus with diabetic peripheral neuropathic pain
- PMID: 39548809
- PMCID: PMC11786186
- DOI: 10.1111/jdi.14355
Blood metabolomic profile in patients with type 2 diabetes mellitus with diabetic peripheral neuropathic pain
Abstract
Aims: This study aimed to identify metabolic markers for diabetic peripheral neuropathic pain (DPNP) in patients with type 2 diabetes mellitus (T2DM).
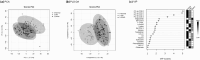
Materials and methods: Blood metabolite levels in the amino acid, biogenic amine, sphingomyelin, phosphatidylcholine (PC), carnitines, and hexose classes were analyzed in nondiabetic control (n = 27), T2DM without DPNP (n = 58), and T2DM with DPNP (n = 29) using liquid chromatography tandem mass spectrometry. Variable importance projection (VIP) evaluation by partial least squares discriminant analysis was performed on clinical parameters and metabolites.
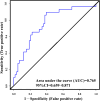
Results: Sixteen variables with VIP > 1.0 (P < 0.05) were identified across all patient groups, and 5 variables were identified to discriminate between the two T2DM groups. DPNP patients showed elevated fasting blood glucose, glutamate, PC aa C36:1, lysoPC a C18:1, and lysoPC a C18:2, while low-density lipoprotein cholesterol, phenylalanine, and tryptophan were reduced. Glutamate, lysoPC a C18:1, and lysoPC a C18:2 discriminated T2DM with DPNP from those without DPNP with an AUC of 0.671. The AUC was improved to 0.765 when ratios of metabolite pairs were considered.
Interpretation: Blood metabolites include glutamate, and phospholipid-related metabolites implicated in neuropathic pain may have the potential as biomarkers for DPNP. Further investigation is required to understand the mechanism of action of these altered metabolites in DPNP.
Keywords: Diabetic peripheral neuropathic pain; Metabolomics; Type 2 diabetes mellitus.
© 2024 The Author(s). Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Approval of the research protocol: Approval was obtained from the ethics committee of Chang Gung Memorial Hospital (IRB No. 201700994B0, 201702035B0, and 201902169B0). The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Informed consent: Individual consent for this retrospective analysis was waived.
Approval date of registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Figures

References
-
- Selvarajah D, Kar D, Khunti K, et al. Diabetic peripheral neuropathy: Advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol 2019; 7: 938–948. - PubMed
-
- Aslam A, Singh J, Rajbhandari S. Prevalence of painful diabetic neuropathy using the self‐completed leeds assessment of neuropathic symptoms and signs questionnaire in a population with diabetes. Can J Diabetes 2015; 39: 285–295. - PubMed
-
- Truini A, Spallone V, Morganti R, et al. A cross‐sectional study investigating frequency and features of definitely diagnosed diabetic painful polyneuropathy. Pain 2018; 159: 2658–2666. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

