The Microbiome Modifies Manifestations of Hemophagocytic Lymphohistiocytosis in Perforin-Deficient Mice
- PMID: 39548906
- PMCID: PMC11739664
- DOI: 10.1002/eji.202451061
The Microbiome Modifies Manifestations of Hemophagocytic Lymphohistiocytosis in Perforin-Deficient Mice
Abstract
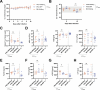
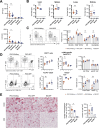
Primary hemophagocytic lymphohistiocytosis (HLH) is a life-threatening hyperinflammatory syndrome caused by inborn errors of cytotoxicity. Patients with biallelic PRF1 null mutations (encoding perforin) usually develop excessive immune cell activation, hypercytokinemia, and life-threatening immunopathology in the first 6 months of life, often without an apparent infectious trigger. In contrast, perforin-deficient (PKO) mice only develop HLH after systemic infection with lymphocytic choriomeningitis virus (LCMV). We hypothesized that restricted microbe-immune cell interactions due to specific pathogen-free (SPF) housing might explain the need for this specific viral trigger in PKO mice. To investigate the influence of a "wild" microbiome in PKO mice, we fostered PKO newborns with Wildling microbiota ('PKO-Wildlings') and monitored them for signs of HLH. PKO-Wildlings survived long-term without spontaneous disease. Also, systemic infection with vaccinia virus did not reach the threshold of immune activation required to trigger HLH in PKO-Wildlings. Interestingly, after infection with LCMV, PKO-Wildlings developed an altered HLH pattern. This included lower IFN-γ serum levels along with improved IFN-γ-driven anemia, but more elevated levels of IL-17 and increased liver inflammation compared with PKO-SPF mice. Thus, wild microbiota alone is not sufficient to trigger HLH in PKO mice, but host-microbe interactions shape inflammatory cytokine patterns, thereby influencing manifestations of HLH immunopathology.
Keywords: Wildling; hemophagocytic lymphohistiocytosis; microbiome; perforin‐deficiency.
© 2024 The Author(s). European Journal of Immunology published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Stepp S. E., Dufourcq‐Lagelouse R., Le Deist F., et al., “Perforin Gene Defects in Familial Hemophagocytic Lymphohistiocytosis,” Science 286, no. 5446 (1999): 1957–1959. - PubMed
-
- Feldmann J., Callebaut I., Raposo G., et al., “Munc13‐4 is Essential for Cytolytic Granules Fusion and is Mutated in a Form of Familial Hemophagocytic Lymphohistiocytosis (FHL3),” Cell 115, no. 4 (2003): 461–473. - PubMed
-
- zur Stadt U., Schmidt S., Kasper B., et al., “Linkage of Familial Hemophagocytic Lymphohistiocytosis (FHL) Type‐4 to Chromosome 6q24 and Identification of Mutations in Syntaxin 11,” Human Molecular Genetics 14, no. 6 (2005): 827–834. - PubMed
-
- Henter J. I., Horne A., Arico M., et al., “HLH‐2004: Diagnostic and Therapeutic Guidelines for Hemophagocytic Lymphohistiocytosis,” Pediatric Blood & Cancer 48, no. 2 (2007): 124–131. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

