Length and Sequence-Selective Polymer Synthesis Templated by a Combination of Covalent and Noncovalent Base-Pairing Interactions
- PMID: 39549037
- PMCID: PMC11613443
- DOI: 10.1021/jacs.4c13452
Length and Sequence-Selective Polymer Synthesis Templated by a Combination of Covalent and Noncovalent Base-Pairing Interactions
Abstract
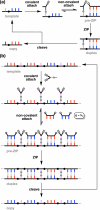
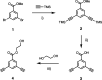
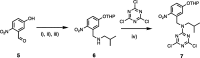
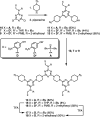
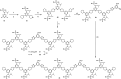
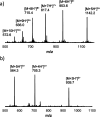
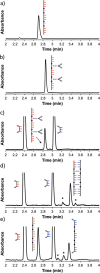
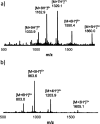
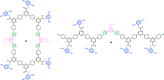
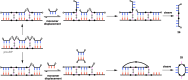
Information can be encoded and stored in sequences of monomer units organized in linear synthetic polymers. Replication of sequence information is of fundamental importance in biology; however, it represents a challenge for synthetic polymer chemistry. A combination of covalent and noncovalent base pairs has been used to achieve high-fidelity templated synthesis of synthetic polymers that encode information as a sequence of different side-chain recognition units. Dialkyne building blocks were attached to the template by using ester base pairs, and diazide building blocks were attached to the template by using H-bond base pairs. Copper-catalyzed azide-alkyne cycloaddition reactions were used to zip up the copy strand on the template, and the resulting duplex was cleaved by hydrolyzing the covalent ester base pairs. By using recognition-encoded melamine oligomers with either three phosphine oxide or three 4-nitrophenol recognition units to form the noncovalent base pairs, exceptionally high affinities of the diazides for the template were achieved, allowing the templated polymerization step to be carried out at low concentrations, which promoted on-template intramolecular reactions relative to competing intermolecular processes. Two different templates, a 7-mer and an 11-mer, were used in the three-step reaction sequence to obtain the sequence-complementary copy strands with minimal amounts of side reaction.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



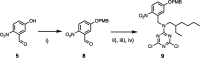



References
-
- Bisson A. P.; Carver F. J.; Hunter C. A.; Waltho J. P. Molecular Zippers. J. Am. Chem. Soc. 1994, 116, 10292–10293. 10.1021/ja00101a056. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

