Exploring mesenchymal stem cells homing mechanisms and improvement strategies
- PMID: 39550211
- PMCID: PMC11631218
- DOI: 10.1093/stcltm/szae045
Exploring mesenchymal stem cells homing mechanisms and improvement strategies
Abstract
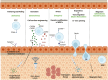
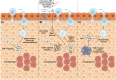
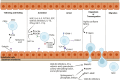
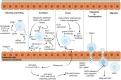
Mesenchymal stem cells (MSCs) are multipotent cells with high self-renewal and multilineage differentiation abilities, playing an important role in tissue healing. Recent advancements in stem cell-based technologies have offered new and promising therapeutic options in regenerative medicine. Upon tissue damage, MSCs are immediately mobilized from the bone marrow and move to the injury site via blood circulation. Notably, allogenically transplanted MSCs can also home to the damaged tissue site. Therefore, MSCs hold great therapeutic potential for curing various diseases. However, one major obstacle to this approach is attracting MSCs specifically to the injury site following systemic administration. In this review, we describe the molecular pathways governing the homing mechanism of MSCs and various strategies for improving this process, including targeted stem cell administration, target tissue modification, in vitro priming, cell surface engineering, genetic modifications, and magnetic guidance. These strategies are crucial for directing MSCs precisely to the injury site and, consequently, enhancing their migration and local tissue repair properties. Specifically, our review provides a guide to improving the therapeutic efficacy of clinical applications of MSCs through optimized in vivo administration and homing capacities.
Keywords: administration; homing; improvement strategies; mesenchymal stem cells; migration; priming; target tissue modification.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
The authors declared no potential conflicts of interest.
Figures



References
-
- Sarukhan A, Zanotti L, Viola A.. Mesenchymal stem cells: myths and reality. Swiss Med Wkly. 2015;145:w14229. https://doi.org/10.4414/smw.2015.14229 - DOI - PubMed
-
- Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP.. Heterotopic transplants of bone marrow. Transplantation. 1968;6:230-247. https://doi.org/10.1097/00007890-196803000-00009 - DOI - PubMed
-
- Campagnoli C, Roberts IA, Kumar S, et al.Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396-2402. https://doi.org/10.1182/blood.v98.8.2396 - DOI - PubMed
-
- Crisan M, Yap S, Casteilla L, et al.A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301-313. https://doi.org/10.1016/j.stem.2008.07.003 - DOI - PubMed
-
- Young HE, Steele TA, Bray RA, et al.Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat Rec. 2001;264:51-62. https://doi.org/10.1002/ar.1128 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

