Tongguanteng injection exerts anti-osteosarcoma effects through the ER stress-associated IRE1/CHOP pathway
- PMID: 39550552
- PMCID: PMC11568601
- DOI: 10.1186/s12906-024-04689-7
Tongguanteng injection exerts anti-osteosarcoma effects through the ER stress-associated IRE1/CHOP pathway
Abstract
Background: In China, Tongguanteng injection (TGT) is widely used in the treatment or adjuvant treatment of various types of cancer. However, the effect and mechanism of TGT in osteosarcoma is not clear.
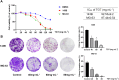
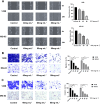
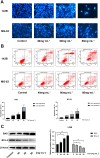
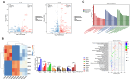
Methods: The 143B and MG-63 cells were treated with different concentrations of TGT. Cell proliferation, migration, invasion and apoptosis were detected using CCK8 assay, transwell assay and flow cytometry. Differentially expressed genes (DEGs) were screened using RNA sequencing (RNA-seq). The identified mRNA and protein expression associated with the IRE1/CHOP pathway was validated by RT-PCR and western blot assay. To explore the underlying mechanisms, 4-phenylbutyric acid (4-PBA) was selected as a specific endoplasmic reticulum (ER) stress inhibitor. Small interfering RNA (siRNA) or pEX-3-ERN1 plasmid was transfected into 143B cells to silence or overexpress IRE1, respectively. The potential downstream proteins, including CHOP, and apoptosis associated proteins, caspase-3 and PARP1 were determined. Furthermore, the effect of TGT was demonstrated in 143B cell tumor-bearing mice in vivo. H&E staining, TUNEL staining and immunohistochemistry were conducted in tumor tissues obtained from the xenograft mouse model.
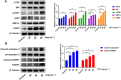
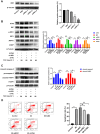
Results: TGT was shown to dramatically suppress the proliferation, migration and invasion, and induce apoptosis of osteosarcoma 143B and MG-63 cells in vitro. The identified DEGs included HSPA5 (encoding BiP) and ERN1 (encoding the IRE1 protein), as well as apoptosis-associated gene DDIT3 (encoding the CHOP protein). The term "IRE1-mediated unfolded protein response" was screened to be the most enriched biological process GO term. The expression of ER stress-associated proteins including ATF6, BiP, p-IRE1, XBP1s and CHOP, as well as apoptosis-associated cleaved caspase-3 and cleaved PARP1 proteins, was significantly upregulated by TGT treatment in osteosarcoma 143B cells, suggesting that TGT might promote the apoptosis of osteosarcoma 143B cells through the IRE1/CHOP pathway. Furthermore, knocking down IRE1 with si-IRE1 or inhibiting of ER stress with 4-PBA suppressed the expression of ATF6, BiP, XBP1s and CHOP induced by TGT, as well as the expression of cleaved caspase-3 and cleaved PARP1. On the contrary, overexpressing IRE1 promoted CHOP expression and induced osteosarcoma cell apoptosis. Consistent with in vitro results, TGT dramatically inhibited the tumor growth and promoted the expression of p-IRE1 and CHOP in tumor-bearing mice.
Conclusion: The findings suggest that TGT exerts an anti-osteosarcoma effect in vitro and in vivo. The underlying mechanism might be associated with the activation of IRE1/CHOP pathway in ER stress. Our findings suggest that targeting IRE1/CHOP pathway might be a potential novel approach for osteosarcoma treatment.
Keywords: Apoptosis; ER stress; IRE1/CHOP pathway; Osteosarcoma; Tongguanteng injection.
© 2024. The Author(s).
Conflict of interest statement
Figures



References
MeSH terms
Substances
Grants and funding
- PKJ2020-Y08/The Science and Technology Development Fund of Shanghai Pudong New Area
- PKJ2023-Y51/The Science and Technology Development Fund of Shanghai Pudong New Area
- 202011/Shanghai Children's Foundation
- 82003987/National Natural Science Foundation of China
- PW2021E-03/Health Industry Special Project of Shanghai Pudong New Area Health Commission
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

