Finerenone, Serum Potassium, and Clinical Outcomes in Heart Failure With Mildly Reduced or Preserved Ejection Fraction
- PMID: 39550716
- PMCID: PMC11571067
- DOI: 10.1001/jamacardio.2024.4539
Finerenone, Serum Potassium, and Clinical Outcomes in Heart Failure With Mildly Reduced or Preserved Ejection Fraction
Abstract
Importance: Treatment with finerenone, a nonsteroidal mineralocorticoid receptor antagonist (MRA), improved outcomes in patients with heart failure with mildly reduced or preserved ejection fraction in FINEARTS-HF, but was associated with increased levels of serum potassium in follow-up.
Objective: To investigate the frequency and predictors of serum potassium level greater than 5.5 mmol/L and less than 3.5 mmol/L and examine the treatment effect associated with finerenone, relative to placebo, on clinical outcomes based on postrandomization potassium levels.
Design, setting, and participants: Secondary analysis of the FINEARTS-HF multicenter, randomized clinical trial, performed between September 14, 2020, and January 10, 2023, with a median follow-up of 32 months (final date of follow-up: June 14, 2024). Patients with heart failure and left ventricular ejection fraction greater than or equal to 40%, New York Heart Association class II to IV symptoms, and elevated natriuretic peptides were included.
Intervention: Participants received finerenone or placebo.
Main outcomes and measures: The primary outcome was a composite of total worsening heart failure events or cardiovascular death.
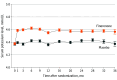
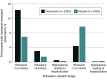
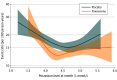
Results: A total of 6001 participants were included (3003 randomized to receive finerenone and 2998 randomized to receive placebo). The increase in serum potassium was greater in the finerenone group than the placebo group at 1 month (median [IQR] difference, 0.19 [0.17-0.21] mmol/L) and 3 months (median [IQR] difference, 0.23 [0.21-0.25] mmol/L), which persisted for the remainder of trial follow-up. Finerenone increased the risks of potassium level increasing to greater than 5.5 mmol/L (hazard ratio [HR], 2.16 [95% CI, 1.83-2.56]; P < .001) and decreased the risks for potassium level decreasing to less than 3.5 mmol/L (HR, 0.46 [95% CI, 0.38-0.56]; P < .001). Both low (< 3.5 mmol/L; HR, 2.49 [95% CI, 1.8-3.43]) and high (>5.5 mmol/L; HR, 1.64 [95% CI, 1.04-2.58]) potassium levels were associated with higher subsequent risks of the primary outcome in both treatment groups. Nevertheless, the risk of the primary outcome was generally lower in patients treated with finerenone compared with placebo, even in those whose potassium level increased to greater than 5.5 mmol/L.
Conclusions and relevance: In patients with heart failure with mildly reduced or preserved ejection fraction, finerenone resulted in more frequent hyperkalemia and less frequent hypokalemia. However, with protocol-directed surveillance and dose adjustment, clinical benefit associated with finerenone relative to placebo was maintained even in those whose potassium level increased to greater than 5.5 mmol/L.
Trial registration: ClinicalTrials.gov Identifier: NCT04435626.
Conflict of interest statement
Figures

Comment in
-
The Fine Art and Science of Translating Trials Results Into Clinical Practice.JAMA Cardiol. 2025 Jan 1;10(1):48-49. doi: 10.1001/jamacardio.2024.4550. JAMA Cardiol. 2025. PMID: 39550722 No abstract available.
References
-
- Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895-e1032. doi: 10.1161/CIR.0000000000001063 - DOI - PubMed
-
- Vardeny O, Claggett B, Anand I, et al. ; Randomized Aldactone Evaluation Study (RALES) Investigators . Incidence, predictors, and outcomes related to hypo- and hyperkalemia in patients with severe heart failure treated with a mineralocorticoid receptor antagonist. Circ Heart Fail. 2014;7(4):573-579. doi: 10.1161/CIRCHEARTFAILURE.114.001104 - DOI - PubMed
-
- Rossignol P, Dobre D, McMurray JJ, et al. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circ Heart Fail. 2014;7(1):51-58. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

