Prenatal exposure to per- and polyfluoroalkyl substances (PFAS) and their influence on inflammatory biomarkers in pregnancy: Findings from the LIFECODES cohort
- PMID: 39550829
- PMCID: PMC11663107
- DOI: 10.1016/j.envint.2024.109145
Prenatal exposure to per- and polyfluoroalkyl substances (PFAS) and their influence on inflammatory biomarkers in pregnancy: Findings from the LIFECODES cohort
Abstract
Background: Per- and polyfluoroalkyl substances (PFAS) are fluorinated chemicals linked to adverse pregnancy and birth outcomes. However, the underlying mechanisms, specifically their effects on maternal inflammatory processes, are not well characterized.
Objective: We examined associations between prenatal PFAS exposure and repeated measures of inflammatory biomarkers, including C-reactive protein (CRP) and four cytokines [Interleukin-10 (IL-10), IL-1β, IL-6, and tumor necrosis factor-α (TNF-α)].
Methods: We analyzed data from 469 pregnant women in a nested case-control study of preterm birth at Brigham and Women's Hospital in Boston, Massachusetts (2006-2008). We measured nine PFAS in early pregnancy plasma samples (median gestation: 10 weeks), with inflammatory biomarkers measured at median gestations of 10, 18, 26, and 35 weeks. We used linear mixed models for repeated measures and multivariable regression for visit-specific analysis to examine associations between each PFAS and inflammation biomarker, adjusting for maternal demographics, pre-pregnancy BMI, and parity. We examined the effects of PFAS mixture using sum of all PFAS (∑PFAS) and quantile-based g-computation approaches.
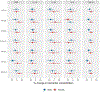
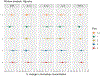
Results: We observed consistent inverse associations between most PFAS and cytokines, specifically IL-10, IL-6, and TNF-α, in both single pollutant and mixture analyses. For example, an interquartile range increase in perfluorooctanesulfonic acid was associated with -10.87 (95% CI: -19.75, -0.99), -13.91 (95% CI: -24.11, -2.34), and -8.63 (95% CI: -14.51, -2.35) percent change in IL-10, IL-6, and TNF-α levels, respectively. Fetal sex, maternal race, and visit-specific analyses showed associations between most PFAS and cytokines were generally stronger in mid-pregnancy and among women who delivered males or identified as African American.
Conclusions: The observed suppression of both regulatory (IL-10) and pro-inflammatory (TNF-α) cytokines suggests that PFAS may alter maternal inflammatory processes or immune functions during pregnancy. Further research is needed to understand the effects of both legacy and newer PFAS on inflammatory pathways and their broader clinical implications.
Keywords: C-reactive protein; Cytokines; Inflammation; LIFECODES; PFAS; Pregnancy.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- National Academies of Sciences E, Medicine, Health, Medicine D, Division on E, Life S, Board on Population H, Public Health P, Board on Environmental S, Toxicology, Committee on the Guidance on PT, Health O: The National Academies Collection: Reports funded by National Institutes of Health. In: Guidance on PFAS Exposure, Testing, and Clinical Follow-Up. Washington (DC): National Academies Press (US); 2022. - PubMed
-
- ACOG: Committee Opinion No 700: Methods for Estimating the Due Date. In: Obstetrics & Gynecology. 129. 2017. e150–e154. - PubMed
-
- Al-Gubory KH, 2016. Multiple exposures to environmental pollutants and oxidative stress: Is there a sex specific risk of developmental complications for fetuses? Birth Defects Res C Embryo Today 108 (4), 351–364. - PubMed
-
- Baines KJ, West RC, 2023. Sex differences in innate and adaptive immunity impact fetal, placental, and maternal healthdagger. Biol Reprod 109 (3), 256–270. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

