Palbociclib in Combination with either Aromatase Inhibitors or Fulvestrant for Patients with Advanced HR+/HER2- Breast Cancer in Germany: Final Results of the Phase 2 Multicohort INGE-B Trial
- PMID: 39551040
- PMCID: PMC11809519
- DOI: 10.1159/000542459
Palbociclib in Combination with either Aromatase Inhibitors or Fulvestrant for Patients with Advanced HR+/HER2- Breast Cancer in Germany: Final Results of the Phase 2 Multicohort INGE-B Trial
Abstract
Introduction: The INGE-B trial (NCT02894398) aimed to confirm the efficacy and safety data from the PALOMA trials for patients treated first line (1L) with palbociclib (PAL) and letrozole or 1L and later line with PAL and fulvestrant. In addition, so far lacking evidence for efficacy and safety on the combination of PAL with anastrozole, exemestane (1L), or letrozole (later line) was investigated.
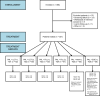
Methods: The prospective, multicenter, multicohort phase 2 trial INGE-B enrolled adult patients with locally advanced, inoperable, or metastatic HR+/HER2- breast cancer in Germany. The primary endpoint was the clinical benefit rate (CBR) in patients with measurable disease according to RECIST v1.1. Secondary endpoints were overall response rate, progression-free survival (PFS), overall survival (OS), safety, and quality of life. Data were analyzed with descriptive statistics.
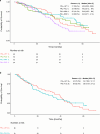
Results: Between 2016 and 2018, 388 patients were enrolled at 64 German sites. Among patients with measurable disease treated with PAL in 1L (n = 157), the CBR was 63.7% (100/157). Among all patients treated with PAL 1L (n = 219), PFS was 20.1 months (95% CI 14.6-24.0), and OS was 40.9 months (95% CI 35.1-49.2). The most common grade 3/4 adverse event was neutropenia (33.4% n = 77). There were no treatment-related deaths.
Conclusion: The INGE-B trial demonstrated good efficacy and tolerability of PAL with letrozole (1L) or fulvestrant (first and later line) in accordance with the PALOMA trials. In addition, the so far lacking proof of efficacy and safety of PAL in combination with anastrozole or exemestane in 1L and with letrozole in later line was provided by INGE-B.
Introduction: The INGE-B trial (NCT02894398) aimed to confirm the efficacy and safety data from the PALOMA trials for patients treated first line (1L) with palbociclib (PAL) and letrozole or 1L and later line with PAL and fulvestrant. In addition, so far lacking evidence for efficacy and safety on the combination of PAL with anastrozole, exemestane (1L), or letrozole (later line) was investigated.
Methods: The prospective, multicenter, multicohort phase 2 trial INGE-B enrolled adult patients with locally advanced, inoperable, or metastatic HR+/HER2- breast cancer in Germany. The primary endpoint was the clinical benefit rate (CBR) in patients with measurable disease according to RECIST v1.1. Secondary endpoints were overall response rate, progression-free survival (PFS), overall survival (OS), safety, and quality of life. Data were analyzed with descriptive statistics.
Results: Between 2016 and 2018, 388 patients were enrolled at 64 German sites. Among patients with measurable disease treated with PAL in 1L (n = 157), the CBR was 63.7% (100/157). Among all patients treated with PAL 1L (n = 219), PFS was 20.1 months (95% CI 14.6-24.0), and OS was 40.9 months (95% CI 35.1-49.2). The most common grade 3/4 adverse event was neutropenia (33.4% n = 77). There were no treatment-related deaths.
Conclusion: The INGE-B trial demonstrated good efficacy and tolerability of PAL with letrozole (1L) or fulvestrant (first and later line) in accordance with the PALOMA trials. In addition, the so far lacking proof of efficacy and safety of PAL in combination with anastrozole or exemestane in 1L and with letrozole in later line was provided by INGE-B.
Keywords: Anastrozole; Endocrine therapy; Exemestane; Fulvestrant; Letrozole; Palbociclib.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
K.P., C.S., L.M., C.B., J.M., A.S., R.L., T.K., H.U.S., G.B., and K.G. declare no conflict of interest concerning the topic of this publication. N.M. is chief executive officer and shareholder of iOMEDICO AG. The institutions of M.W., M.Z., L.M., C.B., C.S., M.U., J.M., D.L., A.W., S.D., V.H., A.S., R.L., and T.K. received remuneration for the documentation of patient data. M.W.: consulting fees: Novartis, Daiichi Sankyo, Lilly, BMS, AstraZeneca; honoraria for lectures/presentations, travel support, participation on a data safety monitoring board, or advisory board: Novartis, Daiichi Sankyo, Lilly, BMS, AstraZeneca, Gilead, Stemline, AbbVie, and Pierre Fabre. M.Z.: honoraria for lectures/presentations: AbbVie, MSD, Takeda, Vifor, Roche, Lilly; participation on a data safety monitoring board, or advisory board: AbbVie, AstraZeneca, BMS, Celgene, Eisai, Gilead, Hexal, Janssen, Lilly, Novartis, Pfizer, Roche, and Vifor. M.U.: honoraria for lectures/presentations: AstraZeneca, Daiichi Sankyo, Lilly, Myriad Genetics, Novartis, Pierre Fabre, Pfizer, Roche, Sanofi Aventis, and Menarini Stemline; participation on a data safety monitoring board or advisory board: AstraZeneca, Daiichi Sankyo, Lilly, MSD Merck, Myriad Genetics, Novartis, Pierre Fabre, Pfizer, Gilead, Roche, Sanofi Aventis Seagen, Menarini Stemline, and CD Pharma. D.L.: consulting fees, honoraria for lectures/presentations, payment for expert testimony, and travel fees: AstraZeneca, Pfizer, Novartis, Eli Lilly, Amgen, Loreal, GSK, onkowissen, high5MD, Daiichi Sankyo, Novartis, and Gilead; leadership or fiduciary role in DGHO from 2012 to 2019. A.W.: advisory boards: MSD, Roche, Novartis, Pfizer, Seagen, Lilly, Menarini Stemline, and P. Fabre; participation on a data safety monitoring board: iOMEDICO; honoraria for lectures/presentations: Roche, Eisai, Novartis, Pfizer, Lilly, iOMEDICO, Interplan, MSD, MCI, RCC, and Menarini Stemline. S.D.: travel support: BeiGene (DGHO 2024); participation on a data safety monitoring board or advisory board: AbbVie, BeiGene, and Servier; stock ownership: iOMEDICO. V.H.: consulting fees: Novartis, Roche, Pfizer, BMS, and Boehringer Ingelheim; honoraria for lectures/presentations: Roche, Amgen, Pfizer, Celgene, BMS, Boehringer Ingelheim, Novartis, AstraZeneca, Lilly, and mteacademy; travel support: Roche, Pfizer, JansenCilag, iOMEDICO, Gilead, Celgene, and Daiichi Sankyo; and stock ownership: BMS, JNJ.
Figures
References
-
- Magge T, Rajendran S, Brufsky AM, Foldi J. CDK4/6 inhibitors: the devil is in the detail. Curr Oncol Rep. 2024;26(6):665–78. - PubMed
-
- AWMF-S3-Leitlinie Mammakarzinom . Interdisziplinäre S3-Leitliniefür die Früherkennung, Diagnostik, Therapie und Nachsorge des Mammakarzinoms. AWMF-Leitlin Regist-Nr 032-045OL Langversion 44. 2021. Available from: https://www.awmf.org/uploads/tx_szleitlinien/032-045OLl_S3_Mammakarzinom...
-
- Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

