Effectiveness and safety of immune checkpoint inhibitors in Black patients versus White patients in a US national health system: a retrospective cohort study
- PMID: 39551068
- PMCID: PMC12169339
- DOI: 10.1016/S1470-2045(24)00528-X
Effectiveness and safety of immune checkpoint inhibitors in Black patients versus White patients in a US national health system: a retrospective cohort study
Abstract
Background: Black patients were severely under-represented in the clinical trials that led to the approval of immune checkpoint inhibitors (ICIs) for all cancers. The aim of this study was to characterise the effectiveness and safety of ICIs in Black patients.
Methods: We did a retrospective cohort study of patients in the US Veterans Health Administration (VHA) system's Corporate Data Warehouse containing electronic medical records for all patients who self-identified as non-Hispanic Black or African American (referred to as Black) or non-Hispanic White (referred to as White) and received PD-1, PD-L1, CTLA-4, or LAG-3 inhibitors between Jan 1, 2010, and Dec 31, 2023. Effectiveness outcomes were overall survival, time to treatment discontinuation, and time to next treatment. The safety outcome was the frequency of immune-related adverse events; assessed among a random sample of 1000 Black patients and 1000 White patients, 892 pairs were matched on the basis of baseline characteristics using 1:1 exact matching without replacement. After manual chart review, patients who did not receive ICI therapy or who had inadequate follow-up were excluded. The adjusted effect of race on each effectiveness outcome was assessed in the whole ICI-treated cohort with propensity-weighted Cox regression with robust standard errors. Immune-related adverse events outcomes were analysed in the random matched sample with multivariable Cox regression, adjusting for baseline characteristics.
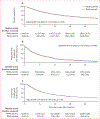
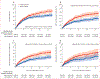
Findings: We identified 26 398 patients, of whom 4943 (18·7%) patients were Black, 21 455 (81·3%) were White, 895 (3·4%) were female, 25 503 (96·6%) were male, 11 859 (45%) had non-small-cell lung cancer, and 26 045 (98·7%) received PD-1 or PD-L1 inhibitors. As of data cutoff (Aug 28, 2024), median follow-up was 40·3 months (95% CI 38·3-42·3) for Black patients and 43·9 months (43·0-45·1) for White patients. Compared with White patients, Black patients had longer time to treatment discontinuation (2-year unadjusted rates 10·7% [95% CI 9·8-11·7] for Black patients vs 8·6% [8·2-9·0] for White patients; adjusted hazard ratio [HR] 0·91, 95% CI 0·87-0·95, p<0·0001), similar time to next treatment (23·5% [22·3-24·8] for Black patients vs 25·6% [25·0-26·2] for White patients; 1·00, 0·95-1·05, p=0·96), and slightly improved overall survival (36·5% [35·2-38·1] for Black patients vs 36·5% [35·8-37·1]; 0·95, 0·90-0·99, p=0·036). 1710 patients (n=862 Black and n=848 White) were analysed for safety outcomes. Compared with White patients, Black patients had a reduced risk of all-grade immune-related adverse events (unadjusted 2-year rate 33·1% [95% CI 28·9-37·1] vs 44·1% [95% CI 39·1-48·7]; adjusted HR 0·75, 95% CI 0·62-0·90, p=0·0026), immune-related adverse events requiring treatment with systemic steroids (0·61, 0·46-0·81, p=0·00051), and immune-related adverse events resulting in permanent ICI discontinuation (0·58, 0·44-0·78, p=0·00024). In exploratory analyses of irAE subtypes, a significant risk reduction in Black patients was found for colitis (0·46, 0·27-0·76, p=0·0026) and hyperthyroidism or hypothyroidism (0·63, 0·44-0·90, p=0·011), and no significant differences were found for any other immune-related adverse event subtypes analysed. Similar results were found in analyses using a steroid-based definition of immune-related adverse events among the entire ICI-treated cohort.
Interpretation: Compared with White patients, Black patients had similar ICI effectiveness and lower toxicities among those treated in the national VHA system, potentially reflecting an important difference in the therapeutic ratio (ratio of benefit to harm) of ICIs. Our findings of decreased toxicity among Black patients require further investigation to assess their generalisability.
Funding: Million Veteran Program, Office of Research and Development, Veterans Health Administration and the LUNGevity foundation.
Copyright Published by Elsevier Ltd.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures

References
-
- Riaz IB, Islam M, Khan AM, et al. Disparities in representation of women, older adults, and racial/ethnic minorities in immune checkpoint inhibitor trials. Am J Med 2022; 135: 984–92. - PubMed
-
- Eggermont AMM, Kicinski M, Blank CU, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: a secondary analysis of a randomized clinical trial. JAMA Oncol 2020; 6: 519–27. - PMC - PubMed
-
- Peltzman T, Rice K, Jones KT, Washington DL, Shiner B. Optimizing data on race and ethnicity for Veterans Affairs patients. Mil Med 2022; 187: e955–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

