Production of site-specific antibody conjugates using metabolic glycoengineering and novel Fc glycovariants
- PMID: 39551135
- PMCID: PMC11697773
- DOI: 10.1016/j.jbc.2024.108005
Production of site-specific antibody conjugates using metabolic glycoengineering and novel Fc glycovariants
Abstract
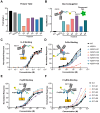
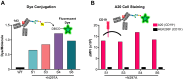
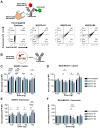
Molecular conjugation to antibodies has emerged as a growing strategy to combine the mechanistic activities of the attached molecule with the specificity of antibodies. A variety of technologies have been applied for molecular conjugation; however, these approaches face several limitations, including disruption of antibody structure, destabilization of the antibody, and/or heterogeneous conjugation patterns. Collectively, these challenges lead to reduced yield, purity, and function of conjugated antibodies. While glycoengineering strategies have largely been applied to study protein glycosylation and manipulate cellular metabolism, these approaches also harbor great potential to enhance the production and performance of protein therapeutics. Here, we devise a novel glycoengineering workflow for the development of site-specific antibody conjugates. This approach combines metabolic glycoengineering using azido-sugar analogs with newly installed N-linked glycosylation sites in the antibody constant domain to achieve specific conjugation to the antibody via the introduced N-glycans. Our technique allows facile and efficient manufacturing of well-defined antibody conjugates without the need for complex or destructive chemistries. Moreover, the introduction of conjugation sites in the antibody fragment crystallizable (Fc) domain renders this approach widely applicable and target agnostic. Our platform can accommodate up to three conjugation sites in tandem, and the extent of conjugation can be tuned through the use of different sugar analogs or production in different cell lines. We demonstrated that our platform is compatible with various use-cases, including fluorescent labeling, antibody-drug conjugation, and targeted gene delivery. Overall, this study introduces a versatile and effective yet strikingly simple approach to producing antibody conjugates for research, industrial, and medical applications.
Keywords: Immunotherapy; antibody engineering; antibody-drug conjugate; glycoconjugate; glycoengineering; glycosylation; immunoglobulin G (IgG); nanoparticle.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests. Z. J. B., K. D. -B., K. J. Y., and J. B. S. are listed as co-inventors on a patent describing the technologies presented herein.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

