Impaired branched-chain amino acid (BCAA) catabolism during adipocyte differentiation decreases glycolytic flux
- PMID: 39551140
- PMCID: PMC11667163
- DOI: 10.1016/j.jbc.2024.108004
Impaired branched-chain amino acid (BCAA) catabolism during adipocyte differentiation decreases glycolytic flux
Abstract
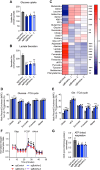
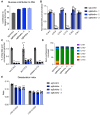
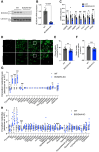
Dysregulated branched-chain amino acid (BCAA) metabolism has emerged as a key metabolic feature associated with the obese insulin-resistant state, and adipose BCAA catabolism is decreased in this context. BCAA catabolism is upregulated early in adipogenesis, but the impact of suppressing this pathway on the broader metabolic functions of the resultant adipocyte remains unclear. Here, we use CRISPR/Cas9 to decrease BCKDHA in 3T3-L1 and human pre-adipocytes, and ACAD8 in 3T3-L1 pre-adipocytes to induce a deficiency in BCAA catabolism through differentiation. We characterize the transcriptional and metabolic phenotype of 3T1-L1 cells using RNAseq and 13C metabolic flux analysis within a network spanning glycolysis, tricarboxylic acid (TCA) metabolism, BCAA catabolism, and fatty acid synthesis. While lipid droplet accumulation is maintained in Bckdha-deficient adipocytes, they display a more fibroblast-like transcriptional signature. In contrast, Acad8 deficiency minimally impacts gene expression. Decreased glycolytic flux emerges as the most distinct metabolic feature of 3T3-L1 Bckdha-deficient cells, accompanied by a ∼40% decrease in lactate secretion, yet pyruvate oxidation and utilization for de novo lipogenesis is increased to compensate for the loss of BCAA carbon. Deletion of BCKDHA in human adipocyte progenitors also led to a decrease in glucose uptake and lactate secretion; however, these cells did not upregulate pyruvate utilization, and lipid droplet accumulation and expression of adipocyte differentiation markers was decreased in BCKDH knockout cells. Overall our data suggest that human adipocyte differentiation may be more sensitive to the impact of decreased BCKDH activity than 3T3-L1 cells and that both metabolic and regulatory cross-talk exist between BCAA catabolism and glycolysis in adipocytes. Suppression of BCAA catabolism associated with metabolic syndrome may result in a metabolically compromised adipocyte.
Keywords: adipogenesis; adipose; branched chain amino acids; glycolysis; metabolic flux.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Boulet M.M., Chevrier G., Grenier-Larouche T., Pelletier M., Nadeau M., Scarpa J., et al. Alterations of plasma metabolite profiles related to adipose tissue distribution and cardiometabolic risk. Am. J. Physiol. Endocrinol. Metab. 2015;309:E736–E746. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

