Inhibition of Cbl-b restores effector functions of human intratumoral NK cells
- PMID: 39551607
- PMCID: PMC11574514
- DOI: 10.1136/jitc-2024-009860
Inhibition of Cbl-b restores effector functions of human intratumoral NK cells
Abstract
Background: T cell-based immunotherapies including immune checkpoint blockade and chimeric antigen receptor T cells can induce durable responses in patients with cancer. However, clinical efficacy is limited due to the ability of cancer cells to evade immune surveillance. While T cells have been the primary focus of immunotherapy, recent research has highlighted the importance of natural killer (NK) cells in directly recognizing and eliminating tumor cells and playing a key role in the set-up of an effective adaptive immune response. The remarkable potential of NK cells for cancer immunotherapy is demonstrated by their ability to broadly identify stressed cells, irrespective of the presence of neoantigens, and their ability to fight tumors that have lost their major histocompatibility complex class I (MHC I) expression due to acquired resistance mechanisms.However, like T cells, NK cells can become dysfunctional within the tumor microenvironment. Strategies to enhance and reinvigorate NK cell activity hold potential for bolstering cancer immunotherapy.
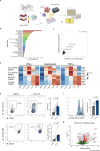
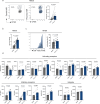
Methods: In this study, we conducted a high-throughput screen to identify molecules that could enhance primary human NK cell function. After compound validation, we investigated the effect of the top performing compounds on dysfunctional NK cells that were generated by a newly developed in vitro platform. Functional activity of NK cells was investigated using compounds alone and in combination with checkpoint inhibitor blockade. The findings were validated on patient-derived intratumoral dysfunctional NK cells from different cancer types.
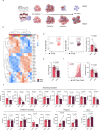
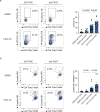
Results: The screening approach led to the identification of a Casitas B-lineage lymphoma (Cbl-b) inhibitor enhancing the activity of primary human NK cells. Furthermore, the Cbl-b inhibitor was able to reinvigorate the activity of in vitro generated and patient-derived dysfunctional NK cells. Finally, Cbl-b inhibition combined with T-cell immunoreceptor with Ig and ITIM domains (TIGIT) blockade further increased the cytotoxic potential and reinvigoration of both in vitro generated and patient-derived intratumoral dysfunctional NK cells.
Conclusions: These findings underscore the relevance of Cbl-b inhibition in overcoming NK cell dysfunctionality with the potential to complement existing immunotherapies and improve outcomes for patients with cancer.
Keywords: Immune Checkpoint Inhibitor; Immunotherapy; Intratumoral; Natural Killer - NK.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: ARom and OG reports personal fees from Hoffmann-La Roche during the conduct of the study and personal fees from Hoffmann-La Roche outside the submitted work. AZ received consulting/advisor fees from Bristol-Myers Squibb, Merck Sharp & Dohme, Hoffmann–La Roche, NBE Therapeutics, Engimmune, and maintains further non-commercial research agreements with Hoffmann–La Roche, T3 Pharma, Bright Peak Therapeutics, AstraZeneca.
Figures
References
-
- Cho BC, Abreu DR, Hussein M, et al. Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol. 2022;23:781–92. doi: 10.1016/S1470-2045(22)00226-1. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
